Published on:
Continuous monitoring of Synthetic Biology
What is new in Synthetic Biology research?
The annual number of publications with new research results has reached a permanently high number. The ZKBS conducts a continuous literature search for the key word "Synthetic Biology". For this, the Pubmed database (https://www.ncbi.nlm.nih.gov/pubmed) is searched for "Synthetic Biology" and periodicals relevant to the newsletter, such as Nature, Science, ACS Synthetic Biology and other subject-oriented newsletters (e.g. The Scientist, SynBioBeta) are sifted through.
The ZKBS regularly selects those among the publications that in their view are particularly relevant to and typical of the individual research fields (Fig. 1) and presents these on their homepage.
The conclusion from the monitoring is that all research approaches as of December 31, 2023 considered here from the research fields of synthetic biology defined by the ZKBS are still regulated by existing legal regulations, in particular the GenTG.
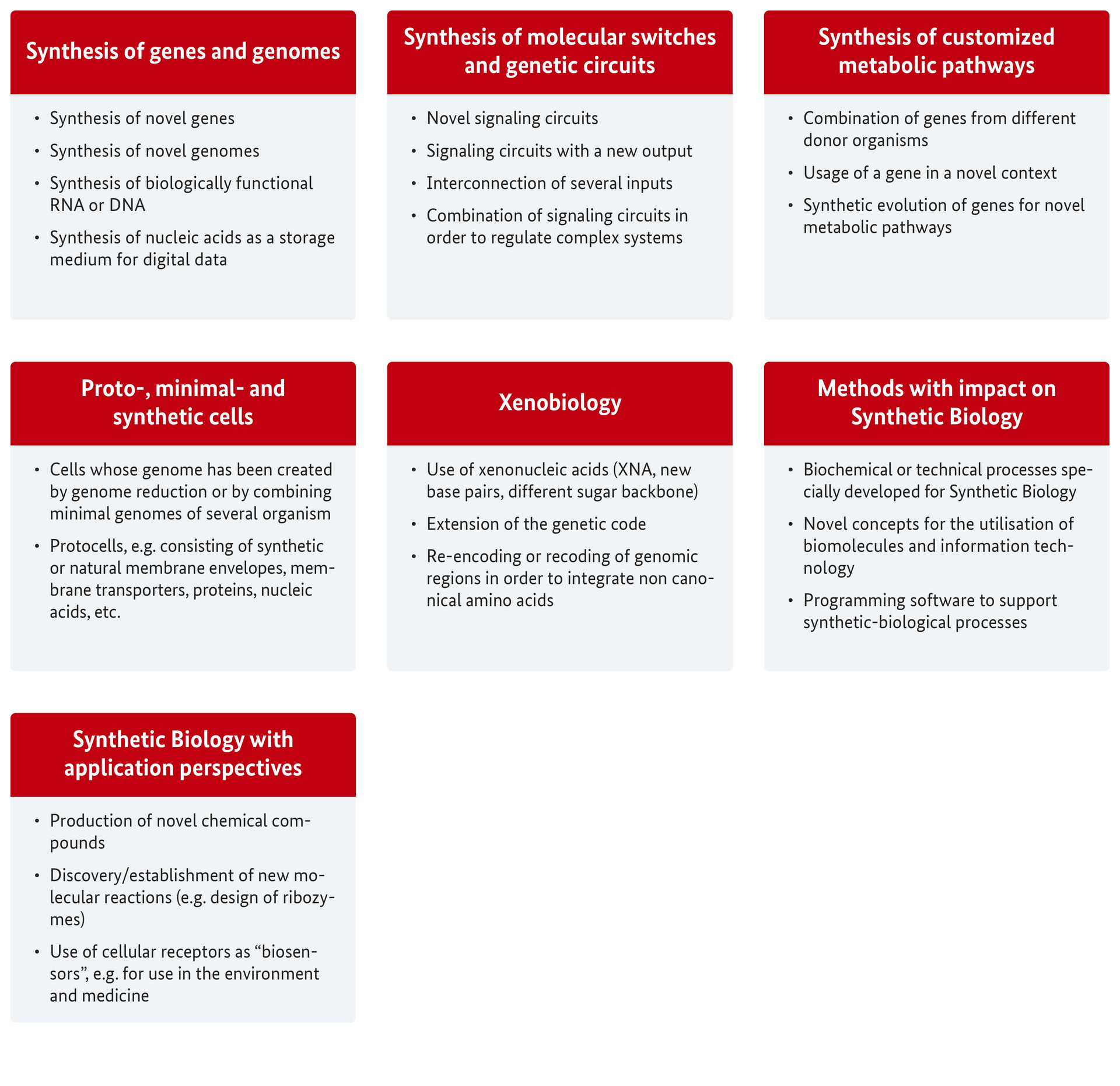
Fig. 1: The research fields of Synthetic Biology
Highlights of Synthetic Biology monitoring of 2023
-
2023
The screening process identified 217 publications that were analysed in more detail. 37 of these now appear as a short summary on the ZKBS-homepage.
The ZKBS considers the work of Inda-Webb et al. to be one highlight paper from 2023. The authors have generated an application which combines biological and technical engineering to a special degree and is therefore a prime example of an application of synthetic biology. The authors have developed a capsule the size of a tablet that contains live bacteria and can be ingested to detect inflammatory markers in the gastrointestinal tract. The information obtained is sent directly to a smartphone. The tablet has already been tested on pigs and could become a valuable diagnostic tool.
Another paper the ZKBS would like to highlight is the work of Belluati et al. Here, the authors have developed a bioreactor in which various biological processes can take place. To produce the bioreactor, the authors used a method in which myoglobin acts as a biocatalyst for the assembly of synthetic polymers into GUVs (giant unilamellar vesicles), i.e. artificial, cell-like structures. This represents a major step towards functional artificial cells.
Monitoring of synthetic biology from 2018 to 2023
Synthesis of genes and genomes
-
2023
Building synthetic chromosomes from natural DNA (Coradini et al. 2023)
The authors report the method CReATiNG (Cloning, Reprogramming, and Assembling Tiled Natural Genomic DNA) for constructing synthetic chromosomes from cloned segments of natural DNA in the yeast S. cerevisiae. This is a faster and cheaper alternative to de novo chromosome synthesis, and could be used in research when complete chromosome reprogramming is not required. In essence, natural chromosome segments are cloned such that unique adapter sequences are appended to their termini, specifying how these molecules will recombine with each other later when they are assembled. Then, cloned segments are co-transferred into cells and assembled by homologous recombination. The method was used to synthetically recombine chromosomes between different strains and species, to modify chromosome structure, and to delete many linked, nonadjacent regions accounting for 39 % of one chromosome. The multiplex deletion experiment reveals that CReATiNG also enables correcting flaws in synthetic chromosome design via recombination between a synthetic chromosome and its native counterpart.Continuous synthesis of E. coli genome sections and Mb-scale human DNA assembly (Zürcher et al. 2023)
The authors developed the tool BASIS, Bacterial artificial chromosome (BAC) Stepwise Insertion Synthesis, a method for megabase-scale assembly of DNA in Escherichia coli episomes. They used BASIS to assemble 1.1 Mb of human DNA. BASIS provides a powerful platform for building synthetic genomes for diverse organisms. The authors also developed the method CGS, Continuous Genome Synthesis, useful for continuously replacing sequential 100 kb stretches of the E. coli genome with synthetic DNA; CGS minimizes crossovers between the synthetic DNA and the genome in a way that the output for each 100 kb replacement provides without sequencing the input for the next 100 kb replacement. Using CGS, a 0.5 Mb section of the E. coli genome was synthesized constituting a key intermediate in its total synthesis from five episomes in 10 days. By parallelizing CGS and combining it with rapid oligonucleotide synthesis and episome assembly and rapid methods for compiling a single genome from strains bearing distinct synthetic genome sections the authors anticipate that it will be possible to synthesize entire E. coli genomes of functional designs in less than two months.Synthetic Yeast Genome Project (Sc2.0)
In this project a global consortium is working to develop the first synthetic eukaryote genome from scratch having a number of specific molecular tags. The final goal of the project is to assemble a fully synthetic yeast organism and to facilitate synthetic biology and engineering research in eukaryotes. Status in 2023: a total of 14 Sc2.0 chromosomes plus a bonus tRNA neochromosome were generated, with two more chromosomes to be constructed. Here, you can find general information and the current status of the Sc2.0 project. In a cluster of papers dedicated to individual synthetic chromosomes, the Sc2.0 consortium describes new findings on aneuploidy, extrachromosomal DNA regulation, chromosome fusion, and many other aspects of yeast genome biology (Blount et al., Foo et al., Lauer et al., Shen et al., Williams et al., Luo et al.). Highlights in this collection include the creation of a yeast strain containing seven synthetic chromosomes, which equals ~ 50 % of the yeast genome, that functions similarly to wild-type yeast (Zhao et al. 2023), a strain with all tRNA genes re-located to an entirely synthetic tRNA neochromosome (Schindler et al. 2023), and the re-design and 3D structural genomic characterization of the largest yeast chromosome (Zhang et al. 2023). -
2022
Multiplex base editing to convert TAG into TAA codons in the human genome (Chen et al. 2022) the authors take the first steps in whole-genome recoding in human cells by demonstrating that the stop codon TAG can be simultaneously converted into TAA in dozens of genes in a single transfection experiment. One goal of whole-genome recoding is to generate virus-resistant cells that could be applicable for biomedicine, especially for making cell therapies or therapeutic production lines resistant to most natural viruses.
The approach is based on previous Escherichia coli recoding projects in which all 314 TAG stop codons were replaced with TAA codons genome-wide (Isaacs et al., 2011). To adapt the approach to the size of the human genome, the authors developed a Python-based software called Genome Recoding Informatics Toolbox (GRIT) that is tailored to recoding and can automate the process of part design. GRIT identified a total of 6700 TAG codons in the human genome of which 1937 sites are located in essential genes and are editable using cytosine base editors.
For the multiplex base editing strategy gBlocks, with each gBlock containing five individual gRNA cassettes, were designed and synthesized. The strategy was optimized by using single-cell RNAseq. Applying 10 gBlocks at a time the authors were able to edit 33 of 47 target sites within the HEK293T cells in a single transfection event while observing ~40 C-to-T off-target events. Nonetheless, this work demonstrates the feasibility of TAG to TAA conservations in the human genome and provides a framework for large-scale engineering of mammalian genomes. -
2021
Imaging cell lineage with a synthetic digital recording system (Chow et al. 2021) the authors developed a system to record different cell lineages in higher organisms by permitting an imaging-based in situ-readout. They used site-specific serine integrases such as Bxb1 for their “integrase-editable memory by engineered mutagenesis with optical in situ readout (intMEMOIR)” system. The cells were marked with a genetic “barcode” flanked by attP and attB sites that allow Bxb1-recombination leading to either a deletion or an inversion of the barcode. The cells´ state can be read out using fluorescence in situ hybridisation (FISH). Ten orthogonal attP and attB sites can be used in the same organism allowing for the recording of 310 states. To test the system in vivo the authors created the Drosophila line memoiphila, which harbours 10 barcodes and whose neuronal cells can be integrase-edited when heat-shocked. Editing was induced four hours after egg laying so that neuroblasts labelled with distinct arrays can pass on their editing to all progeny in the adult brain. Adult flies were then dissected and their brains subjected to several rounds of FISH analysis reading out the induced editing as well as the expression of eight endogenous genes that mark different neuronal cell types. The combination of gene expression and cell clones identified by barcode editing allowed to determine several known cell types. The possibility to visualize the cell lineage directly in the native tissue could provide insights into the roles of different factors in cell fate development.
-
2020
Belcher et al. (2020) built libraries of synthetic promoters and corresponding transcription factors (TFs) for plants. These shall enable a more precise control of transgene expression and thus provide for easier engineering in plants. The authors started with a plant minimal promoter to which they added different concatenated cis-elements from the well-characterised yeast GAL4 regulon. These cis-elements are bound by the GAL4 TF. The cis-element-containing promoters showed different expression strengths that allowed to be used for tuning gene expression. 25 such promoters were successfully tested in vivo in Arabidopsis thaliana. The number of TF was then expanded to TFs from other families and their respective cis-elements. Finally, transactivators and repressors were added to the promoter library by fusing known transactivation domains from Zea mays or Herpes simplex virus type 1 or the repressor domain SRDX to the TFs or truncated versions thereof that consisted only of the DNA-binding domain. The hybrid promoters bind to different TFs coupled to different activator or repressor domains, thus allowing multi-gated logic operations in plant cells. - to the original literature
Kotopka et al. (2020) established an in silico based method to generate artificial promoters for Saccharomyces cerevisiae. In order to generate the basic data for this method, conserved motifs from known promoters in combination with randomized spacer sequences were used to create two plasmid based libraries in S. cerevisiae. These libraries comprise over 675,000 constitutive and over 327,000 promoters inducible by an artificial transcription factor (ZEV). The yeast libraries were analysed by fluorescence-activated cell sorting combined with high-throughput DNA sequencing (FACS-seq). In the next step an in silico model was build, predicting promoter activity as a function of sequence. By implementing a convolutional neural network (CNN) as a deep learning technique, the model was able to handle the very large data sets. To validate the predictions made with this model, sets of artificial promoters were generated in silico with three different sequence designs: 1) screening: generation of random sequences based on the original libraries, 2) evolution: mutagenesis of specific sequences and 3) gradient ascent: iterative modification of initially random sequences. In addition, a GC constraint was implemented when estimating promoter activities. These sets of artificial promoters were then tested against control-promoter-sets by transformation of yeast and FACS-seq analysis. The analysis revealed that screening and evolution strategies produced promoter sets with comparable diversity to the strongest promoters in the initially generated data. The gradient ascent promoter strategy led to a loss in diversity. Further optimisation of evolution and gradient ascent strategies finally led to high-performing promoter sequences even when applying a GC content constraint. This model can be used to not only generate sequences with useful rare properties but also large and sequence-diverse sets of promoters exhibiting high activities. - to the original literature
Lin et al. (2020) established a single strand (ss) double strand (ds) DNA architecture for DNA-based dynamic operations and reusable information storage (DORIS). Systems to access information from DNA data storage should heed three basic criteria: 1) scalability, 2) compatibility with efficient and dense encodings and (3) reusability. In order to meet these criteria dsDNA with a single-stranded overhang (ss-dsDNA) were generated by PCR and hybridization. The overhang sequence („file address“, 20 nt) was followed by the dsDNA containing the T7 promotor (23 nt) and the encoded data (data payload, 117 nt) were designed and generated by PCR. In this design, all strands encoding for one specific file have the same „file address“. By using oligonucleotides complementary to the „file address“ and coupled to magnetic beads, it was possible to separate file related strands out of a pool of mixed DNA molecules under room temperature conditions. In contrast to PCR-based separation methods, the data payload remains annealed under these conditions, blocking any undesired oligonucleotide binding to any similar sequences in the data payload regions. Since unwanted binding is blocked, the sequence of the data payload can be more flexible, thereby increasing density (information per nt) and capacity (storage maximum of the system) of DORIS. To perform as a storage device, DORIS was designed to be capable of in-store file operations such as locking, unlocking, renaming, and deleting files. In a proof of principle, high temperatures (98°C) were used to place a so-called lock, a 20 nt long complementary oligonucleotide, on the sequence of the „file address“. To unlock the system, a key-oligonucleotide, which is complementary to the lock, was added. Renamings of files were performed by oligonucleotides consisting of the merged sequence of the new „file address“ and the old „file address“, creating a new strand overhang. Oligonucleotides blocking the „file address“ were used to delete files from the storage. These in-storage modifications combined with the increased density and capacity of the system are a first step towards DNA based highly parallel processing of extreme levels of information (e.g. medical, genomic and financial data). - to the original literature
-
2019
Fredens et al. (2019) have synthesized the biggest synthetic genome known so far, a 4 Mbp Escherichia coli-genome. They used the REXER (replicon excision for enhanced genome engineering through programmed recombination) technique to exchange the whole length genome of E. coli MDS42 with a synthetic genome. To create this synthetic genome, only 61 of the possible 64 codons were used in protein-coding genes. Two codons for serine (TCG, TCA) and one stop codon (TAG) were replaced with synonymous codons, resulting in the change of 18,214 codons in total. - to the original literature
Wang et al. (2019) developed and tested a system for the precise allocation of the unmodified genome of E. coli to defined, circular artificial chromosomes. The subdivision of E. coli genome thereby took place in the cell by means of Cas9, the lambda-red recombination system and an artificial bacterial chromosome (BAC), which functioned as an acceptor for a part of the genome. A reduced genome-chromosome and a BAC chromosome with the residual genome thus resulted. The subdivision of the genome had only a small influence on the growth behaviour of cells and remained stable across generations. Additional modifications of the system permitted undertaking targeted inversions or translocations in the separated genome and to combine defined sections of genomes of various E. coli strains with one another. As a result, a system was created that made rapid, precise engineering of large genomes possible. - to the original literature
Synthesis of molecular switches and genetic circuits
-
2023
Programmable mammalian translational modulators by CRISPR-associated proteins (Kawasaki et al. 2023)
The authors repurposed Cas proteins as translational modulators in mammalian cells to build artificial logic circuits. A Cas-binding motif was introduced into the 5‘ untranslated region of a gene of interest. When the respective Cas-protein was present in the same cell, the translation of the gene of interest was repressed by the binding of Cas to the binding-motif present in the mRNA. They also constructed an ON switch, in which the Cas-protein binding prevents the mRNA from non-sense mediated decay. Using a split-Cas9, genetic logic gates (NAND) were constructed as well.Engineered bacterial swarm patterns as spatial records of environmental inputs (Doshi et al. 2023)
The authors have modified Proteus mirabilis, a bacterium that naturally forms centimetre-sized bullseye swarm patterns on solid agar, to respond to specific environmental stimuli and form characteristic swarm patterns. These swarm patterns serve as a visual representation of environmental conditions and allow complex environmental information to be captured and visualised in a simple manner. Specifically, a P. mirabilis strain was developed that visually recorded the presence of up to 50 mM copper in water samples, with a graded change from 0 mM to 50 mM in the ring widths and colony radii at the spot locations. This work provides an approach for the construction of macro-scale bacterial recording devices.Tailored Synthetic sRNAs Dynamically Tune Multilayer Genetic Circuits (Velazquez Sanchez et al. 2023)
The authors have designed novel small RNAs (sRNAs) that dynamically modulate gene expression of genetic circuits with a broad spectrum of repression (high, medium, and low), by utilising the intrinsic RNA interference pathway in E. coli. The sRNAs were designed to bind to their respective target sequences with different binding affinities, whereby the binding energy correlates with the repression strength. The sRNAs do not bind directly to the mRNA of the target gene, but to the start codon of the mRNA of a transcription factor that controls the corresponding target gene. The results suggest that gene expression can be dynamically modulated across different conditions by incorporating synthetic sRNA to existing genetic constructs without modification of the genetic parts, e. g. exchanging promoters.Co-opting signalling molecules enables logic-gated control of CAR T cells (Tousley et al. 2023)
The authors developed a chimeric antigen receptor (CAR) engineering approach in which the traditional CD3ζ domains for T cell activation were replaced by intracellular proximal T cell signalling molecules. It is shown that some proximal signalling molecules such as ZAP-70 and PLCγ1 after their conversion as surface receptors are themselves sufficient to initiate CAR T cell signalling and eradicate tumours in vivo without the need for CD3ζ. The main role of ZAP-70 is to phosphorylate LAT and SLP-76, which form a scaffold for signalling propagation. The co-operative role of LAT and SLP-76 has been exploited to develop a logic-gated intracellular network (LINK) CAR, a rapid and reversible Boolean-logic AND-gated CAR T cell platform that outperforms other systems in both efficacy and prevention of on-target, off-tumour toxicity. LINK CAR will broaden the range of molecules that CAR T cells can target and will enable these powerful therapeutics to be used in solid tumours and various diseases such as autoimmunity and fibrosis.Engineering a modular double-transmembrane synthetic receptor system for customizing cellular programs (Zhou et al. 2023)
The authors developed a synthetic receptor system based on two receptor domains, one of which carries a protease and the other a synthetic transcription factor (TF) which is released after cleavage by the protease. The receptor chains are located at the extracellular side and dimerize after binding a ligand, whereupon the protease is able to release the TF, which then can transcribe the customised downstream genes. The receptor system was further engineered to be sensitive to extracellular protein signalling with minimal background signals and positive loop switching. It is shown that this synthetic receptor system can be easily customised to respond to various inputs such as interleukin-1 (IL-1), programmed death ligand 1 (PD-L1) and HER2 and to release tailored outputs, including fluorescent signals and the therapeutic molecule IL-2.Conditional Control of Universal CAR T Cells by Cleavable OFFSwitch Adaptors (Kvorjak et al. 2023)
The authors have refined universal chimeric antigen receptors (CARs). Universal CARs provide better control over T cell function by not binding the target antigen directly compared to conventional CAR T cells, but instead the CAR binds to a co-administered antibody adaptor that is specific for the target antigen. The authors have further improved the universal CARs by developing OFF-switch adapters. These carry a cleavable biotin tag that can be removed and thus controlled by the addition of a small molecule (phosphine-2-(diphenyl-phosphanyl)-benzamide (2DPBM)) or a light stimulus (UV light). In addition, OFF-switch adapters were able to simultaneously control several antigens orthogonally according to Boolean logic in adaptor combination tests. OFF-switch adaptors represent a new approach for the precision targeting of universal CAR T cells with potential for enhanced safety. -
2022
A synthetic switch based on orange carotenoid protein to control blue-green light responses in chloroplasts (Piccinini et al. 2022) the authors have developed a synthetic orthogonal photoreceptor in plant chloroplasts whose spectral range does not overlap with endogenous plant photoreceptors. They split the orange carotenoid protein (OCP) 2 from cyanobacterium Fischerella thermalis, which is activated by blue-green light, into two domains. The domains can join when a prosthetic keto-carotenoid is present and their interaction was detected by fusing them to split nanoluciferase fragments that produce a fluorescent signal when interaction occurs. The photoreceptor was functional in Arabidopsis thaliana plants that also expressed the bacterial enzyme β-carotene ketolase from Agrobacterium aurantiacum in its chloroplast to provide the prosthetic keto-carotenoid. The system was then applied to regulate transcription of plastid genes by fusing the OCP2 domains to the Sigma2 factor, which is part of the plastid-encoded polymerase, and an anti-sigma factor from T4 phage. That way, the transcription of a plastid gene was inhibited when the two OCP2 domains joined in the dark and was activated by exposure to green light. The photoreceptor can be used to control gene expression in prokaryotes and eukaryotes upon blue-green light exposure.
Photoswitching of feedback inhibition by tryptophan in anthranilate synthase (Bhagat et al. 2022) the authors created a system to control feedback inhibition of an enzyme by light. In feedback inhibition an enzyme of a biosynthetic pathway is inhibited when the end-product binds to an allosteric site. Here, the anthranilate synthase from Salmonella typhimurium was used as a proof-of-concept. The enzyme catalyses the first step in the tryptophan-producing pathway and its synthase subunit TrpE is feedback inhibited by tryptophan. The authors inserted the bulky noncanonical amino acid o-nitrobenzyl-O-tyrosine (ONBY) into TrpE by expanding the genetic code. ONBY possesses an o-nitrobenzyl caging group that hinders the binding of tryptophan. This inhibition can be reversed by irradiation with UV that removes the o-nitrobenzyl and allows tryptophan binding to its allosteric site. Such systems can be used for light-dependent activation or inactivation of enzymes in different biological applications such as biotherapeutics.
Synthetic genetic circuits as a means of reprogramming plant roots (Brophy et al. 2022) the authors have developed a set of transcriptional regulators to build genetic circuits in plants, that will allow the specific expression of genes in different cell types to control plants´ responses to a changing environment. They constructed transcriptional activators, repressors and activatable and repressible synthetic plant promoters. Activators were composed of a DNA-binding domain, an activation domain and a nuclear localisation signal (NLS) and repressors of DNA-binding proteins and a NLS. For an activatable promoter one to six copies of the DNA sequence bound by the transcription factors were fused to a minimal cauliflower mosaic virus (CaMV) 35S promoter, the repressible promoter was made up of a full-length CaMV 35S promoter and one DNA sequence fused to its 3´ end. In the model plant Arabidopsis thaliana the synthetic input transcription factors were placed under control of different tissue-specific promoters allowing the circuits to produce different spatial pattern of gene expression in the plant root. As an application, the authors modified the plants´ lateral root branch density. They designed a logic gate to express a mutated gene called slr-1 that eliminates root branching only in lateral root stem cells where the generally negative side-effects of this mutation are not observed. The gates allowed for an expression of the mutated gene at varying levels and thus for a predictable lateral root development.
Spatiotemporal control of engineered bacteria to express interferon-γ by focused ultrasound for tumor immunotherapy (Chen et al. 2022) the authors have developed a system to control the spatiotemporal expression of therapeutic genes in bacteria that target and reduce tumors. They introduced the gene for the cytokine interferon-γ (IFN-γ) into Escherichia coli MG1655 and placed it under control of the temperature-regulated leftward and rightward phage lambda promoter. When delivered into tumor-bearing mice the bacteria can be heated with ultrasound to stimulate the expression of IFN-γ. The ultrasound-activated bacteria can penetrate into deep-seated tumors and are non-invasively activated by ultrasound.
Tunable and modular miRNA classifier through indirect associative toehold strand displacement (Chen & Chen 2022) the authors use microRNAs (miRNAs) as inputs for logic-gated classifiers. They refined the catalytic hairpin assembly (CHA), in which two complementary strands are prepared as hairpins so that their interaction is blocked. If a single-stranded nucleic acid such as a miRNA is added, the two hairpins are opened successively. The strand displacement is detected by a reporter duplex consisting of a fluorophore and a quencher strand, that will emit fluorescence if the fluorophore strand is displaced by the opened hairpin. By changing the lengths of toeholds and adding variable clamps to the hairpin strand, any miRNA could be adapted to the system. The system was designed as AND, NOT, and ANDNOT gates with up to four inputs and can be used as a simple diagnostic assay.
Computing within bacteria: programming of bacterial behaviour by means of a plasmid encoding a perceptron neural network (Becerra et al. 2022) the authors showed that trained neural networks can be implemented in silico in a colony of synthetic bacteria. Without performing wet-lab experiments, they programmed bacteria with different neural networks either solving an optimization problem or responding to changing environmental conditions. The neuronal network was first tested offline, implemented into a genetic circuit using the programming language Gro and finally integrated into a plasmid using the Cello platform and the programming language Verilog. The authors conclude that the use of algorithms from computer science in synthetic biology will give bacteria artificial intelligence to solve a range of different problems from medicine to bioremediation.
Orthogonal control of gene expression in plants using synthetic promoters and CRISPR-based transcription factors (Kar et al. 2022) the authors have constructed three mutually orthogonal synthetic plant promoters. They used a minimal Cauliflower mosaic virus (CaMV) 35S promoter and added synthetic guideRNA-binding sequences that can be activated through a guideRNA and a dCas9:VP64 transcription factor. When putting gRNA expression under control of the endogenous signalling molecule ethylene, circuits can be tied to the cellular metabolism. Plant expression vectors to test the promoters were constructed with the modular cloning framework MoClo and tested in Nicotiana benthamiana and Arabidopsis thaliana. The orthogonal promoters can be used for computing operations in plants limiting crosstalk with endogenous pathways and could also be applied to non-model plants.
Developments of mammalian cell logic gates controlled by unnatural amino acids (Mills et al. 2021) the authors used genetic code expansion in mammalian logic gates. They expanded the genetic code by incorporating unnatural amino acids (UAA) into proteins by using a quadruplet genetic code. Therefore, they screened 11 quadruplet-decoding pyrrolysyl tRNA variants described in the literature for Escherichia coli for their function in mammalian cells and identified the variants decoding CUAG or AGGA as functional. For the construction of a double-input logic gate, an E. coli derived tyrosin aminoacyl-tRNA synthetase/tRNA pair was identified as a second orthogonal pair. Both pairs (PylRS/tRNA and TyrRS/tRNA) functioned orthogonally in the same cell and incorporated their specific UAA. The two codons for the pairs were inserted in two split GFP polypeptides that need to assemble for fluorescence to construct AND and OR gates in which either both or at least one of the UAA has to be incorporated to reconstitute the GFP fluorescence. These gates are an alternative approach for logic gate construction in mammalian cells and employ biologically inert molecules.
A logically reversible double Feynman gate with molecular engineered bacteria arranged in an artificial neural network-type structure (Srivastava and Bagh 2022) the authors built an artificial neural network (ANN) that acts as a double Feynman gate. The double Feynman gate has three inputs (X1, X2, X3) and three outputs (O1, O2, O3) and one input leads to one defined output. O1 is a control and is defined as O1 = X1. O2 is defined as X1 + X2 and O3 as X1 + X3. The in- and outputs are calculated as logical operations in so-called truth tables that address a weight to each individual input/output combination and together built five artificial neuro-synapses. These logic operations were implemented into Escherichia coli bacterial cells as bactoneurons where extracellular chemical inducers act as inputs and fluorescent proteins as outputs. Weights are applied as gene activation or repression of synthetic promoters and biases were added. The authors constructed and co-cultured five bactoneurons (bacterial cultures) and obtained a double Feynman gate function at the population level. This kind of ANN-type architecture can be used for cellular computation operations.
Synthetic multistability in mammalian cells (Zhu et al. 2022) the authors created multistability, in which genetically identical cells can exist in molecularly distinct and mitotically stable cell states, with the help of a circuit architecture composed of transcription factors (TF). These TF can heterodimerize competitively and will only activate their own genes as homodimers (auto-activation). The authors first developed a set of zinc finger transcription factors with homodimer-dependent self-activation and heterodimer-dependent inhibition. When stably integrated into mammalian cell lines, the cells would express either one of the TF or both thus exhibiting three different states. The states could be changed by adding external inducers. When protein stability was reduced, the cells transitioned from tristable to bistable. The system was then expanded by adding a third transcription factor, which resulted in cells exhibiting seven distinct states. An expansion beyond three TF was modelled and seems to be possible. The paper is a step towards understanding and using multistability, for example for engineered cell therapies.
-
2021
LED control of gene expression in a nanobiosystem composed of metallic nanoparticles and a genetically modified E. coli strain (Aratboni et al. 2021) the authors have established a photothermal control of gene expression using non-toxic metallic nanoparticles that convert light into heat. Free conducting electrons in gold nanoparticles (AuNPs) oscillate upon illumination and part of this energy is emitted as heat increasing the temperature of the surrounding medium. The authors activated AuNPs by a light-emitting diode in microorganisms expressing the fluorescent protein mCherry under control of a U6 RNA thermometer. RNA thermometers can be naturally found in the 5´-untranslated regions of mRNAs and control the expression of downstream genes by temperature-induced changes in their conformation. At low temperatures the RNA thermometer masks the ribosome binding site, at higher temperatures the RNA secondary structure melts locally and allows for ribosome binding. It was shown that mCherry was only expressed at room temperature when the AuNPs were illuminated. This work provides another option to control gene expression in biological systems by illumination.
De novo design of a reversible phosphorylation-dependent switch for membrane targeting (Harrington et al. 2021) the authors developed a minimal de novo peptide-based molecular switch that is based on a heterodimeric coiled-coil assembly and changes between monomer and dimer upon phosphorylation and dephosphorylation. When coupled to a membrane-targeting peptide the switch can shift between a membrane bound and a solution state. Such a switch can be used for transcriptional regulation and construction of orthogonal, protein-based circuits.
T cell circuits that sense antigen density with an ultrasensitive threshold (Hernandez-Lopez et al. 2021) the authors designed T cell circuits to prevent „off-tumour“ toxicities, frequently occurring during cancer treatment with chimeric antigen receptor (CAR)-T cells. The authors envisioned CAR-T cells which reliably distinguish cancer cells from normal cells by antigen density and enable an increased cancer cell killing. Using the human epidermal growth factor receptor 2 (HER2) as a target, ultrasensitive T cells with a two-step recognition circuit were designed by combining a synNotch receptor with a CAR. The synNotch receptor detects the antigen with low affinity, thereby acting as a high-(antigen)density-filter. When the synNotch receptor becomes activated it induces the expression of a high-affinity CAR which upon activation induces T cell proliferation and T cell mediated killing of tumour cells. The designed T cells were tested on human leukaemia (K562) cells, expressing different amounts of HER2, for their ability to discriminate between cells expressing >106.5 and 104.5 HER2 molecules per cells. These HER2-expression levels correspond to those on HER2-expressing cancer cells and normal HER2-expressing tissues respectively. The T cells were able to meet the designated discrimination parameters. In a mixed culture, with high-density (107) and low-density (104.8) HER2 cells, the designed T cells selectively eliminated cells with the high HER2 density, leaving the low-density cells unharmed. To evaluate how these T cells behave in a more complex environment, immunocompromised mice were injected subcutaneously with a high-HER2-density K562 tumour in one flank on the one side and a low-HER2-density K562 tumour in the other flank. After the tumours were established, the designed T cells were administered and showed a strong discrimination: while high-density tumours were cleared from mice, low-density tumours retained growth. This ultrasensitive antigen-density discrimination provides a very important tool for treating solid tumours with T cells.
Spatiotemporally confined red light-controlled gene delivery at single-cell resolution using adeno-associated viral vectors (Hörner et al. 2021) the authors engineered an adeno-associated viral (AAV) vector system for the transfer of genetic information into cells controlled by illumination with red light. AAV vectors transduce both dividing and non-dividing cells and are widely used as gene therapy vehicles. The light-control of the systems controls transduction at the level of cell entry. The AAV vector is engineered in a way that it cannot recognize its natural cell entry receptor heparin sulfate proteoglycan, but expresses the phytochrome-interacting factor 6 (PIF6) from Arabidopsis thaliana. To enable interaction with the target cell an adapter protein is needed. The adapter protein consists of phytochrome B of A. thaliana and a designed ankyrin repeat protein specific for a cell surface protein. Upon illumination with red light, the phytochrome B part of the adapter protein interacts with PIF6 on the viral vector to recruit the vector to the target cell and initiate transduction. Illumination with far-red light dissociates the viral vector from the cell. By switching the adapter protein different cell types can be targeted and the system could easily be applied, for example, to in vivo gene therapy.
An engineered protein-phosphorylation toggle network with implications for endogenous network discovery (Mishra et al. 2021) the authors developed a bistable toggle switch consisting of reversible protein-protein phosphorylation interactions. The switch contains two branches that repress each other and two inputs to switch between the two states of the system. The protein network is built in S. cerevisiae from eleven proteins either stemming from the endogenous MAKP-pathway or being constructed as exogenic chimeric proteins combining endogenous protein domains with domains from Arabidodsis thaliana and Mus musculus. The interaction between the proteins is regulated by phosphorylation: the protein of one branch will interact with the protein of the next branch it represses only when it is phosphorylated. The resulting toggle network is sensitive, can respond quickly to the input signals, and shows long-term bistability across cell divisions. It was shown to control outputs such as fluorescence or abrogation of cell division. Furthermore, the authors developed a computational algorithm that identified 109,401 potential endogenous bistable pathways. 186 of these pathways were tested and five exhibited bistability unknown before. The protein-based toggle-switch could be used as a sensitive environmental sensor or a micro-electronic device for delivery into the gut where it could detect medical conditions such as internal bleeding.
Light-controllable RNA-protein devices for translational regulation of synthetic mRNAs in mammalian cells (Nakanishi et al. 2021) the authors created a system for optogenetic control of mRNA translation of modified RNAs, in which modified nucleosides were incorporated. The authors developed two light-responsive activation systems based on the caliciviral VPg-based translational activator (CaVT). One system is a split CaVT, in which the RNA binding domain and the translational activation domain can only interact if a ligand is uncaged by light. The second system uses a destabilizing domain-fused CaVT that is rapidly degraded if the ligand is absent and is stabilized if the ligand is uncaged by light. The split CaVT has the advantage to be more robust towards shorter exposure times with the uncaged ligands, while the destabilizing domain-fused CaVT is capable not only of activation but also of repression. The controllable expression of modified RNAs will be useful for gene therapy as these RNAs show enhanced translation efficiency, decreased immunogenicity and cytotoxicity and no risk of insertional mutagenesis.
Wearable materials with embedded synthetic biology sensors for biomolecule detection (Nguyen et al. 2021) the authors embedded cell-free, freeze-dried genetically engineered polynucleotide circuits in materials such as textiles or silicones creating wearable sensors for the detection of small molecules, nucleic acids or toxins. They first established a sensor with the lacZ-operon as an output that was used to detect different inputs: (1) detection of anhydrotetracycline via a transcription factor-regulated circuit, (2) detection of Ebola virus RNA via a toehold switch and (3) detection of the small molecule theophylline via a riboswitch. Sensor circuits were also embedded in fibre optic textiles to allow the detection of viral (HIV) or bacterial (Borellia burgdorferi) RNA via a fluorescent or luminescent output. A sensor based on a programmable CRISPR system was used for direct nucleic acid detection. In this sensor, a Cas12a protein-gRNA combination detects a target dsDNA and subsequently cleaves a quenched ssDNA fluorophore probe resulting in a fluorescent output. The CRISPR-system was successfully applied to the detection of Staphylococcus aureus resistance markers. The authors also used their system to construct a sensor integrated into a face mask for the detection of SARS-CoV-2 RNA that was as sensitive as the standard laboratory RT-PCR assay.
-
2020
Chen et al. (2020) explored the possibility of designing logic gates using de novo designed protein heterodimers. In order to build such logic gates, they first defined specific properties of protein building blocks: 1) high number of orthogonal pairs to prevent gate complexity limitations, 2) blocks to be modular and similar in structure to simplify gate construction, 3) single blocks to be able to bind to different partners with different and tuneable affinities, 4) the interaction of the blocks to be cooperative to ensure that gate activation is not sensitive to stoichiometric imbalances in the inputs. To meet these properties in their design, Chen et al. used existing datasets of orthogonal designed heterodimers (DHD) which all share the same four-helix-structure. The monomers were fused with flexible linkers in a way that the interaction domains (surfaces) are buried within the fusion domains and would require free energy to get exposed. This type of construction ensures that the logic gate is only activated by the sum of the binding energy of cooperating DHD pairs. Purified circuit elements were used to demonstrate the functionality of the DHD logic gates under variable experimental conditions in a cell-free system and in yeast cells, using gene expression control of a fluorescent reporter as a read out. Additionally, multi input gates based on DHD have been tested as well, revealing the system’s scalability. As a proof of concept, Chen et al. aimed to repress the immune checkpoint gene for the T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) in native T-cells. Therefore, an “OR” DHD logic gate was constructed, in which one monomer of the DHD is linked to the transcription activator like effector (TALE) DNA-binding domain and the other to the Krüppel-associated box (KRAB) repressor domain. If the monomers are brought into proximity to each other, the expression of TIM3 could be repressed. Thus, a protein logic gate was designed that, due to its flexibility, composition, and scalability, could enable post-translational control over a variety of biological functions. - to the original literature
Frei et al. (2020) generated miRNA-based incoherent feedforward (iFF) circuits to rescue the expression level of genes of interest despite changes in endogenous cellular conditions. Thereby, this work contributes to the development of robust-by-design mammalian synthetic circuits. To generate the necessary data for designing the iFF circuits, Frei et al. started by investigating the burden imposed by transiently expressed synthetic circuits on mammalian cells. It was shown that exogenous gene expression in HEK293T and H1299 cells had a negative impact on host cell protein expression. Additionally, the authors demonstrated that miRNA mediated downregulation can help mitigating the metabolic burden of a genetic circuit. Based on these data, a model for synthetic circuits was established, in which expression of the gene of interest is uncoupled from endogenous environmental changes. The model was able to adapt to existing circuit topologies such as the open-loop and iFF loop to include pools of shared and limited resources. As a proof of concept, an iFF circuit with a miRNA mediated downregulation using miRNA target sites complementary to an endogenous or synthetic miRNA was tested in silico and in vitro. As expected, the iFF circuit was less sensitive to changes in available resources. - to the original literature
Krawczyk et al. (2020) have linked electrical stimulation of human cells to either transgene expression or secretion of protein therapeutics from intracellular vesicles. The electronic information is converted into protein production and release via depolarization of the cell membrane. The authors introduced L-type voltage-gated calcium channels into the membrane of mammalian cells. The channels open when the membrane is depolarized by an electric pulse, enabling a calcium influx which finally leads to the induction of target genes by the nuclear factor of activated T cells (NFAT). As a proof of concept, human β cells that secrete insulin upon calcium influx were made electrosensitive by expressing the ion channel. Such cells were placed into a bioelectronic implant that can be stimulated wirelessly. When implanted into type 1 diabetic mice, the electrosensitive cells were able, upon electrical stimulation, to secrete insulin and decrease blood glucose levels to normal. - to the original literature
Lajoie et al. (2020) designed colocalization-dependent protein switches (Co-LOCKR) that perform AND, OR, and NOT Boolean logic operations to enable a specific cell targeting. For example, the system can be used to recognize a specific antigen combination on tumour cells to induce a directed anti-tumour action. Based on the original design of the LOCKR-Switch (Langan et al. 2019), where a protein cage is kept inactive by a latch protein until a key protein binds and enables the interaction with the effector protein, a new LOCKR-version with shorter helices, an improved hydrophobic packing and an additional hydrogen bond network was generated. To target cells with a combination of specific surface antigens, targeting domains were added that recruit the Co-LOCKR cage and key proteins to a cell expressing specific target antigens. The authors showed that the cage and key could be recruited to Her2- and EGFR-expressing cells by the targeting domains in an AND logic. When adding a second key, OR logic was introduced. A NOT logic was established by adding a decoy protein acting as a molecular sponge for the key and fused to a targeting domain against a surface marker to be avoided. In a proof of principle experiment the authors generated chimeric antigen receptor (CAR)-T-cells targeting an exogenously expressed protein on cells. The CAR-T-cells were specifically directed towards tumour cells expressing Her2 and EGFR when adding the Co-LOCKR, the key, and the targeting domain. In experiments, in which CAR-T cells were co-cultured with AND/OR or AND/NOT Co-LOCKRs and tumour cells displaying the designated antigen, the Co-LOCKR carried out the expected logic, resulting in T-cell proliferation at a specific antigen combination. Co-LOCKR was able to identify a specific tumour cell line in a co-culture of different tumour cells based on the antigen combination on its cell surface. - to the original literature
Pinto et al. (2020) characterized a library of 34 split inteins (internal proteins). Inteins are auto-catalytic protein segments excising themselves from a larger precursor peptide whose flanking residues are then ligated through the formation of a new peptide bond, a process known as protein splicing. The intein itself is excised. Split inteins are inteins joining two protein halves that are expressed from different genes. The authors used a split mCherry protein to evaluate the cis-splicing (both protein halves are on the same plasmid) and trans-splicing (protein halves and split inteins are on different plasmids) capacity of split inteins under similar conditions. The authors found 15 mutually orthogonal split intein pairs capable of cis- and trans-splicing of which 10 could be simultaneously used in a cell-free assay. The split inteins were then used for logic circuit operations, two-input/two-output as well as three-input/three-output logic circuits, where they were coupled to split transcriptional regulators. In another application, split inteins were used to assemble large repetitive proteins from multiple peptides and may thus be applied in biomaterial manufacturing. - to the original literature
Wiechert et al. (2020) built and tested synthetic promoters for precise inducible expression systems based on the bacterial mechanism of xenogenic silencing and counter-silencing. Xenogenic silencers are nucleoid-associated bacterial proteins that preferentially binding to horizontally acquired AT-rich DNA. The authors used the xenogenic silencer CspS, a prophage-encoded protein from Corynebacterium glutamicum. CspS acts as a silencer when it binds to a target promoter, oligomerizes and forms a nucleoprotein complex inhibiting transcription. To regulate this inhibition a synthetic counter-silencer was included in the promoter sequences, the operator site of the transcription factor GntR. When GntR binds to the synthetic counter-silencer promoter it interferes with the nucleoprotein complex allowing transcription to start. 44 synthetic counter-silencer promoters were identified. In addition, the system was used to build a toggle switch in C. glutamicum switching between cell growth and L-valine production.- to the original literature
Williams et al. (2020) have worked on combinatorial antigen pattern recognition on tumour cells to enable more specific cancer-cell targeting. The authors used multiple synthetic Notch (synNotch) receptors to flexibly link a range of receptors and outputs into circuits. In a three-input-AND gate, the first synNotch receptor recognises antigen 1, becomes activated and induces the expression of a second synNotch receptor. If this synNotch receptor is activated by the recognizing antigen 2, a chimeric antigen receptor (CAR) is expressed that binds the third antigen and leads to target cell killing. As an alternative to the three-input-AND-gate, the authors also tested a three-input AND-NOT gate, in which a synNotch leads to activation of a CAR and a third synNotch receptor, if activated, induces the expression of a proapoptotic protein. In this circuit, the presence of only the first two antigens leads to the killing of a tumour cell, whereas the presence of antigen 3 will destroy the T cell itself. - to the original literature
-
2019
Aoki et al. (2019) - A closed biological control circuit was integrated into E. coli cells for the first time, based on the engineering principle of integral regulators. For this, Bacillus subtilis-σ- and anti-σ-factors (SigW and RsiW) were used. SigW activates the expression of araC and the gene of interest (GOI), which should be held stable by the control circuit. The anti-σ-factor RsiW binds and inactivates SigW, so that less araC and GOI are expressed. Because RsiW is simultaneously under the control of an araC dependent promoter, a lower araC concentration also leads to less RsiW. From this, a control circuit resulted that also withstood disturbances such as temperature variations in experiments. As proof of principle the authors used the methionine-synthetase gene as the GOI, producing an enzyme that is essential in methionine-free media for the growth of the cells. The control circuit led to a stable growth rate of cells. - to the original literature
Chung et al. (2019) developed a genetic circuit that detects a protein (ErbB) specifically overexpressed on the surface of tumor cells and thereupon induces apoptosis in those cells. The system consists of two proteins: a protease, which is fused to a phosphotyrosine-binding domain (PBD), and an intracellular membrane associated carrier-protein with flexible cargo. The protease only binds constitutively phosphorylated ErbB, which is found mainly in cancer cells, and thus gets in close proximity to the cell membrane. The protease is then able to cleave off the cargo and e. g. induce apoptosis. As a proof of concept the signaling circuit has been introduced into healthy hepatocytes and pancreatic cancer cells with an adeno-associated viral vector, resulting in a cancer cell-restricted apoptosis. - to the original literature
Huang et al. (2019) - Oncolytic viruses are increasingly be used in the therapy of solid tumours. In order to achieve a higher tumour specificity and an improved effect Huang et al. created programmable oncolytic adenoviruses. These contain a circuit, comprising a tumour-specific promoter, a tumour- specific microRNA and a microRNA expressed mainly in healthy cells. If the tumour-specific promoter is activated, the tumour-specific microRNA increases and the microRNA specific to healthy cells is little present, the adenovirus expresses the factor E1 and an immune effector. An improved effect and increased tissue specificity for hepatocellular carcinoma was thereby achieved. - to the original literature
Langan et al. (2019), Ng et al. (2019) - To implement a synthetic feedback control for endogenous signal paths and synthetic genetic circuits, in the first step a de novo protein system (LOCKR switch) was developed, which can undergo conformational changes (Langan et al.). The system comprises a bundle of six helices, of which five form a protein cage and the sixth helix represents a protein trap with integrated peptide sequences to bind the target molecule. In the initial conformation the sixth helix is bound to the cage and the trap is inaccessible for the target molecule. The addition of a key that is homologous competitive to the cage is associated with a change in the conformation of the helices, whereby the binding site of the target molecule is exposed such that the protein trap becomes functional. Building upon this system, Ng et al. implemented a synthetic feedback control for endogenous signal paths and synthetic genetic circuits. In the two-part work, a degronLOCKR switch (Langan et al.) was used first in order to modulate the native MAPK signal cascade in yeast such that an increased or diminished output of the signal cascade can be induced. The second part of the work concerns the use of the degronSwitch in synthetic genetic circuits. For this, a simple hormonally influenced, synthetic transcriptional cascade in yeast was tested with and without feedback-control, comprised of two inducible degronSwitch-merged transcription factors (GEM, Z3PM9). In a direct comparison with the circuit without feedback control, the circuit with feedback control was more adaptable to outside influences (hormone concentration changes) and thus represents the basis for implementing complex synthetic functions in cells. - to the original literature: Langan et al. (2019), Ng et al. (2019).
Liao et al. (2019) - Complex circuits are often lost in cells through evolutionary processes, because they are associated with a loss of fitness. In order to avoid this loss, the authors use a co-culture with three sequentially employed E. coli strains that respectively include a toxin-antitoxin system (TA-system). In addition to the respective individual TA system, such a system also codes for an antitoxin for one of the other strains. It is thereby assured that bacteria without the suitable antitoxin in the co-culture are rapidly killed and replaced by those with the antitoxin. The newly added bacterial strain with the antitoxin is thus resistant compared to the previously grown bacteria and can now dominate the culture. In a rotation principle, the second strain could then be replaced by a third and this, in turn, through the first, before mutations lead to undesirable effects. Through this principle the second circuit, which is contained in all three strains on the same plasmid as the TA system, remains stably maintained in the culture. As proof-of-principle, a circuit for population-dependent lysis was used that, at a sufficiently high concentration of a quorum sensing molecule, triggers the lysing of cells. In this circuit high selection pressure normally prevails, so that the cells adapt after two days and lysis no longer takes place. The rotation principle in the TA system allowed the formation of mutants to be prevented and the longevity of the circuit to be clearly extended. - to the original literature
Pandi et al. (2019). In order to generate advanced synthetic biological circuits, Pandi et al. first developed combined transducer-actuator-models in silico and tested these in Escherichia coli. These models consist of a transducer layer containing the gene for an enzyme converting an input molecule such as hippurate or cocaine into the output molecule benzoate and an actuator layer in which benzoate directs the expression of an output signal such as GFP. Two or more transducers can be combined into an analogous, metabolic concentration adder when the genes for two or more converting enzymes, e.g. hippurate- and benzaldehyde-converting enzymes, are placed in the same operon. The fluorescent output then will increase if either metabolite’s concentration is increased. The transducer-actuator system was also tested in a cell-free system, in which the transducers can be weighted by adding different concentrations of DNA. The more enzyme coding DNA was added, the more GFP-fluorescence was observed, demonstrating a direct correlation. This weighted transducer-actuator system was then defined as a single layer metabolic perceptron. The perceptron is a biological computation algorithm that mimics the neuron’s ability to process information, learn, and make decisions. Like a neuron, a metabolic perceptron should be able to receive multiple input signals in the form of different substrates and, as an output, trigger the specific transcription of a target gene, depending on the weighted sum of the inputs. The perceptron per se is a binary classifier, meaning: if the calculated weighted sum of the inputs reaches a predefined threshold, the decision ON is reached and fluorescence is established, otherwise the systems decision is OFF. The authors created a metabolic 4-input binary logic gate perceptron that can classify different amounts of input substrates by applying different weights to each transducer. In vitro experiments confirmed that the in silico model accurately predicted weights to obtain the full OR logic gate behavior with short execution times. This work combines analog information processing with a digital output, thus laying the groundwork for more advanced metabolic circuits for rapid and scalable multiplex sensing. - to the original literature
Saltepe et al. (2019) - Nanoparticles (NP) are playing an increasingly greater role in clinical applications and can, among others, be used to transfer chemotherapeutic agents into tumour tissue. In the development of new NP, above all the cytotoxicity and the biocompatibility are of fundamental significance. To generate a reliable and rapid toxicity sensor for a first test for NP compound screening, Saltepe et al. established a genetic circuit in E. coli, based on the heat shock response mechanism (HSR) of Mycobacterium tuberculosis. This mechanism reacts to the cellular stress induced by toxic NP with the activation of the HSR promoter and the expression of a downstream fluorescent reporter gene. In the result, a test was created that makes rapid, simple optical selection possible. - to the original literature
-
2018
Purcell et al. (2018) present two approaches to encrypt synthetic gene circuits in order to protect the user’s intellectual property. The major challenge is to prevent the decryption of the organization of the synthetic gene circuit through full genome sequencing. The first strategy is based on site-specific unidirectional recombinases and their recognition sites to scramble circuit topology, while the circuit is dormant. A second approach follows the incorporation of additional genes, which are irrelevant for the gene circuit but serve as a camouflage. Furthermore, Purcell et al. present a “molecular key” to decrypt the data by removing or repressing the effect of the proteins expressed by the camouflage genes, leading to correctly reassembled data. As an example, the key could be a plasmid, containing components for a CRISPR-interference, enabling the repression of camouflage gene expression. - to the original literature
Segall-Shapiro et al. (2018) designed an incoherent feed-forward loop with a stabilized promoter to enable a constant gene expression decoupled from internal and external influences, such as plasmid copy number or genomic localization. The system shall be used to stabilize the expression of different genes in customized synthetic pathways. Since cells are subject to dynamic changes during growth or differentiation, stable gene expression after introduction of a new pathway can be challenging and generally needs laborious adjustments. To regulate the expression of the gene of interest (GOI), Segall-Shapiro et al. implemented a repressor that binds to the GOI´s promoter. The gene encoding this constitutively expressed repressor is cloned upstream of the GOI, ensuring that both genes are equally affected by changes in the host cell. The GOI is thus expressed independently of copy numbers. plasmid copy numbers. - to the original literature
Toda et al. (2018) present a synthetic network of cell-cell communication that creates customized complex as well as asymmetric multicellular structures, the basis of any synthetic self-organizing tissue and bio-materials. The approach follows a synthetic signaling circuit based on the synNotch juxtacrine signaling platform. Within the circuit specific cell-cell-contacts change the cadherine cell adhesion, resulting in cell differentiation and production of further cell-cell signals. - to the original literature
Synthesis of customized metabolic pathways
-
2023
ATP production from electricity with a new-to-nature electrobiological module (Luo et al. 2023)
The authors constructed a new-to-nature electrobiological module, the acid/aldehyde ATP cycle (AAA cycle) that enables a cell to directly convert electrical energy into ATP. The AAA cycle consists of four enzymes and does not require a membrane-based charge separation. It can be coupled to other in vitro processes such as transcription/translation systems. The conduction of electrons into the module is accomplished by hexamethyl viologen, enabling the direct storage of electricity as energy in biological systems.Complete integration of carbene-transfer chemistry into biosynthesis (Huang et al. 2023)
The authors integrated the unnatural carbene transfer reaction for the first time into a biosynthetic pathway of a bacterium. The carbene transfer reaction is a prime example of a reaction that is available to synthetic chemists but has been lacking in biology, resulting in a narrower range of accessible products in biosynthesis than in synthetic chemistry. The biosynthetic gene cluster for the natural α-diazoester azaserine from Streptomyces fragilis is introduced into Streptomyces albus. The authors show that azaserine serves as a carbene donor to cyclopropanate, an intracellularly produced styrene, to generate unnatural amino acids with a cyclopropyl group. The reaction is catalysed in S. albus by an evolved, mutant cytochrome P450 enzyme with its native cofactor. The study creates a scalable microbial platform for performing intracellular abiological carbene transfer reactions to functionalise a range of natural and new-to-nature products and expands the range of organic products that can be produced by cellular metabolism.Manipulation of sterol homeostasis for the production of 24-epi-ergosterol in industrial yeast (Jiang et al. 2023)
The authors constructed a yeast cell factory for the scalable production of 24-epi-ergosterol. 24-epi-ergosterol is an unnatural sterol that can be used as a precursor for the semi-synthesis of brassinolide, a plant hormone that has the potential for a wide range of agricultural applications limited by its extremely low natural occurrence and the lack of synthetic precursors. The authors constructed the artificial metabolic pathway by first introducing a Δ24(28)-sterol reductase (DWF1) from plants in Saccharomyces cerevisiae, followed by enzyme-directed evolution to enhance the catalytic activity of DWF1 and enable de novo biosynthesis of 24-epi-ergosterol. The sterol fluxes towards 24-epi-ergosterol were further strengthened by the engineering of sterol homeostasis, via overexpression of three endogenous genes (YEH1, YEH2, and ARE2). The sterol homeostasis engineering strategy can be used for mass production of other economically important phytosterols.Construction and modular implementation of the THETA cycle for synthetic CO2 fixation (Luo et al. 2023)
The authors have constructed a new-to-nature CO2-fixation pathway, the THETA (tricarboxylic acid branch/4-hydroxybutyryl-CoA/ethylmalonyl-CoA/acetyl-CoA) cycle. The THETA cycle comprises 17 enzymes from 9 organisms and is centred around two of the most efficient CO2-fixing enzymes described in nature, crotonyl-CoA carboxylase/reductase and phosphoenolpyruvate carboxylase. Rational and machine learning-based optimisation approaches have improved the yield of the cycle by two orders of magnitude and demonstrated the formation of various biochemical building blocks directly from CO2. The THETA cycle was divided into three modules that were implemented in vivo in Escherichia coli. This represents the first step towards the realisation of highly orthogonal and complex CO2-fixation pathways in the background of living cells.Construction of an artificial phosphoketolase pathway that efficiently catabolizes multiple carbon sources to acetyl-CoA (Yang et al. 2023)
The authors have designed and constructed an artificial phosphoketolase (APK) pathway that consists of only 3 types of biochemical reactions and has the potential to achieve 100% carbon yield to acetyl-CoA from any monosaccharide. The central enzyme in this pathway is phosphoketolase, while phosphatase and isomerase act as auxiliary enzymes. For the conversion of fructose-6-phosphate the APK pathway included phosphoketolase (PK) from Actinobacteria bifidobacterium (BbPK), haloacid dehalogenase (HAD)-like hydrolase (EcHAD) from E. coli, and L-rhamnose isomerase (Ps-LRhI) from Pseudomonas stutzeri, while for the conversion of xylulose-5-phosphate BbPK, triose phosphate isomerase (EcTIM) from E. coli, sugar phosphatase from Candida parapsilosis (CpHAD), and formolase (FLS), a synthetic enzyme, were involved. The APK pathway was tested in vitro and it was shown that typical C1-C6 carbohydrates were efficiently metabolised to acetyl-CoA with a yield of 83 % to 95 %. In addition, the authors have developed E. coli strains that are able to grow via the APK pathway when glycerol is used as a carbon source. The novel APK pathway has the potential for using multiple carbon sources with higher efficiency in biomanufacturing in the future.Engineering α-carboxysomes into plant chloroplasts to support autotrophic photosynthesis (Chen et al. 2023)
The authors generated morphologically correct carboxysomes in tobacco chloroplasts by transferring to them the genetic components from the well-studied α-carboxysome of proteobacterium Halothiobacillus neapolitanus. The fully functional α-carboxysome consists of components encoded by nine carboxysome genes and replacing the endogenous tobacco rbcl gene that encodes the ribulose-1,5-biphosphate carboxylase/oxygenase (Rubisco), an important enzyme involved in carbon fixation. α-carboxysome supports tobacco plant growth and autotrophic photosynthesis at elevated CO2 levels. This work paves the way to improve crop plant photosynthesis and productivity.Engineering a new-to-nature cascade for phosphate-dependent formate to formaldehyde conversion in vitro and in vivo (Nattermann et al. 2023)
The authors developed a new pathway to reduce formate to formaldehyde using two enzymes and inorganic phosphate (Pi). First, formate is phosphorylated and activated to formyl phosphate with acetate kinase from Escherichia coli followed by reduction to formaldehyde with a variant of N-acetyl-γ-glutamyl phosphate (NAGP) reductase (ArgC) from the bacterium Denitrovibrio acetiphilus. Using a semirational enzyme engineering approach, the authors have developed a novel formyl phosphate reductase variant from ArgC. The enzyme is a triple substitution variant of the enzyme that exhibits a complete loss of native enzyme activity (i. e. NAGP reduction), a 300-fold shift in specificity towards formyl phosphate and reduced side reactivity with acetyl phosphate. The functionality of the pathway was demonstrated both in vitro and in vivo in E. coli. In addition, the Pi-based route was linked to a recently developed formaldehyde assimilation pathway, the FORCE pathway (Chou et al. 2021). This ultimately enables the production of C2 compounds in vitro and in vivo from formate as the sole carbon source. -
2022
A biobricks metabolic engineering platform for the biosynthesis of anthracyclinones in Streptomyces coelicolor (Wang et al. 2022) the authors have found a way to synthesize clinically relevant anthracyclines from the actinomycete Streptomyces coelicolor. They created a genetic toolbox called BIOPOLYMER (BIOBricks POLYketide Metabolic EngineeRing) consisting of engineered strains, vectors, promoters, and biosynthetic genes for the synthesis of anthracyclinones. They engineered four different anthracyclinone pathways and explored their effectivity. BIOPOLYMER is thought to serve as a platform for the synthesis of designer anthracycline analogues.
Conversion of CO2 into organic acids by engineered autotrophic yeast (Baumschabl et al. 2022) the authors used the synthetic autotrophic yeast strain Komagataella phaffii (Pichia pastoris) described by Gassler et al. as a host to engineer organic acid producing strains. In a previous study the K. phaffii strain was provided with the Calvin-Benson-Bessham cycle, enabling it to grow continuously with CO2 as a sole carbon source. In the present work two genes from Aspergillus terreus, a cis-aconitate decarboxylase (cadA) and a mitochondrial tricarboxylic acid transporter (mttA), were introduced into the autotrophic K. phaffii strain for the production of itaconic acid. For lactic acid synthesis ldhL from Lactobacillus plantarum was implemented into the K. phaffii genome and the CYB2 gene was deleted by using CRISPR-Cas9. The resulting strains are able to produce organic acids solely from CO2 as carbon source. They could be used for the production of value-added chemicals and act as CO2-neutral or CO2-negative chassis organisms.
-
2021
Microbial synthesis of vanillin from waste poly(ethylene terephthalate) (Sadler & Wallace et al. 2021)theauthors designed an engineered Escherichia coli that converts post-consumer plastic waste into vanillin. The polyethylenglycol (PET)-derived monomer terephthalate (TA) was shown to be converted into vanillin in a 5-step enzymatic pathway, consisting of enzymes from bacteria (Comamonas sp., Nocardia iowensis and Bacillus subtilis) and a mammal (Rattus norvegicus, rat).
A new-to-nature carboxylation module to improve natural and synthetic CO2 fixation (Scheffen et al. 2021) theauthors engineered a new-to-nature reaction for the CO2-dependent assimilation of the C2 compound glycolate into the central carbon metabolism without carbon loss. The few natural pathways converting C2 into C3 metabolites, e. g. photorespiration, result in carbon loss. The authors engineered the so far hypothetical tartronyl-CoA (TaCo) pathway, a synthetic photorespiratory bypass that represents an alternative to natural photorespiration. In this pathway, glycolate is metabolized to glycerate by a three-enzyme-reaction transforming glycolate into glycolyl-CoA, then into tartronyl-CoA, and finally into glycerate. An engineered glycolyl-CoA synthetase from Erythrobacter sp., NAP1, was found to catalyse the first reaction, while a malonyl-CoA reductase from Chloroflexus aurantiacus catalyses the third step and converts tartronyl-CoA into glycerate. The key enzyme of the pathway, a glycolyl-CoA carboxylase (GCC), had yet to be identified. The authors screened propionyl-CoA carboxylases for their potential as a GCC, because they exhibit a similar structure. They identified the propionyl-CoA carboxylase of Methylorubrum extorquens as a candidate, mutated the gene and applied a directed evolution approach to obtain a protein with similar efficiency to naturally occurring biotin-dependent acyl-CoA carboxylases.
The synthetic GCC enzyme proved to be functional in in vitro experiments in combination with the two other enzymes of the TaCo pathway and could be combined with other synthetic CO2 fixation cycles such as the CETCH (crotonyl-coenzyme A (CoA)/ethylmalonyl-CoA/hydroxybutyryl-CoA) cycle.Optogenetic control of plant growth by a microbial rhodopsin (Zhou et al. 2021)the authors established an optogenetic control system in plants which is able to control their growth. Microbial rhodopsins are proteins often expressed as optogenetic tools, but are difficult to express in plants due to a lack of the essential cofactor retinal. The authors used the green light-gated anion channel-rhodopsin ACR1 from the unicellular algae Guillardia theta in tobacco plants. To provide the necessary retinal, the bacterial ß-carotene 15,15`-dioxygenase MbDio as a fusion protein with the chloroplast transit peptide RC2, that allows retinal location to the chloroplast, was co-expressed. The expression of these proteins allowed for a green light-induced anion efflux through the plants´ cell membrane and membrane potential depolarization.
In a proof-of-principle experiment, the authors used the MbDio-RC2-ACR1-construct to test the hypothesis that pollen tube growth is controlled by a local anion channel activation and the resulting voltage gradient. While global illumination led to a massive anion efflux which either did not affect or stopped the tube growth, local illumination changed the tube´s growth direction away from the side in which ACR1 was activated, thereby confirming the hypothesis. This work provides the basis for a powerful tool in studying signalling in plants and could also enable the testing of chemical-electrical signalling. -
2020
Miller et al. (2020) developed a semi-synthetic chloroplast by encapsulating and operating photosynthetic membranes in cell-sized droplets. In order to establish a module for the light-driven regeneration of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADP+), thylakoid membranes from the chloroplasts of Spinacia oleracea were isolated and initially tested for their ability to perform a light-dependent reduction of NADP+ to NADPH and regeneration of ATP from adenosine diphosphate (ADP). Since the thylakoid membranes were able to produce NADPH and ATP as predicted, Miller et al. used these membranes not only to provide energy for individual enzymatic reactions for CO2-fixation but also for a complete synthetic metabolic cycle for the continuous fixation of CO2. This synthetic metabolic cycle consists of three different parts: 1) the artificial 16 enzyme crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA (CETCH) pathway for in vitro fixation of CO2, 2) the glyoxylate/hydroxypyruvate reductase from Escherichia coli and 3) a crotonase from Pseudomonas aeruginosa. In order to create a semi synthetic chloroplast, a microfluidic platform was developed, in which the metabolically active microcompartments can be automatically assembled into water-in-oil micro droplets. The optimisation of the droplet resulted in a complex system that not only possesses essential characteristics of photosynthesis but also outperforms systems using only single enzymes in energy production. This work provides the basis for the development of a completely self-sustaining synthetic organelle. - to the original literature
Reifenrath et al. (2020) use endoplasmic reticulum-derived vesicles to compartmentalise the enzymes of a metabolic pathway into membrane surrounded organelles. That way, potential toxic side effects of unwanted reactions caused by a heterologously expressed protein can be avoided. The three enzymes of the cis,cis-muconic acid (CCM) pathway (toxic for yeast) were fused to a synthetic peptide containing the self-assembling region of the maize storage protein gamma-Zein (Zera). Zera can induce so-called protein bodies derived from the endoplasmic reticulum or the vacuole in plants as well as in heterologous systems. The Zera-induced vesicles with heterologous CCM-enzymes were generated in the yeast Saccharomyces cerevisiae and the functionality of the metabolic pathway in the vesicles was shown. - to the original literature -
2019
Gleizer et al. (2019) - The authors have produced an E. coli strain that, for the first time, uses CO2 as the sole source to produce biomass. The conversion of atmospheric CO2 into foods, fuels and biochemicals should thereby be possible. For carbon fixation the bacteria use the Calvin cycle, whereby the electrochemically produced molecule formate (HCOO-) serves to gain energy. The Calvin cycle drives the conversion of formate into ATP, converting CO2 into sugar and other organic molecules. The autotrophy was achieved through evolution under laboratory conditions: the xylose concentration was reduced in a chemostat, while adequate formate and CO2 were available. The bacteria became autotrophs through various mutations, of which many were in genes with a metabolic connection to the Calvin cycle. - to the original literature
Luo et al. (2019) established a complete biosynthetic pathway for the production of several complex cannabinoids in the yeast Saccharomyces cerevisiae. To achieve this the mevalonate-pathway of yeast was modified by introducing genes from different organisms and Cannabis sativa necessary for the hexanoyl-CoA pathway into the yeast genome. The genetically modified yeast enables a controlled industrial production of cannabinoids. - to the original literature
South et al. (2019) increased growth and productivity of the C3 plant tobacco by introducing a synthetic, more effective photorespiration pathway. When facing high temperatures and dry periods, C3 plants like wheat, rice and soybean increasingly produce glycolate, which is toxic for the plant and has to be converted to non-toxic molecules by photorespiration. This process can reduce the harvested biomass by up to 50 %. By introducing a synthetic glycolate pathway in the chloroplast and simultaneously inhibiting the native photorespiration pathway, biomass production in homozygous transgenic lines was increased by up to 40 %. - to the original literature
Proteo-, minimal- and synthetic cells
-
2023
Artificial cell synthesis using biocatalytic polymerization-induced self-assembly (Belluati et al. 2023)
The authors have developed a more robust biocatalytic polymerization-induced self-assembly (bioPISA) for the generation of giant unilamellar vesicles (GUVs) in order to synthesize cell-like structures. Myoglobin was selected as biocatalyst because of its facilitation of atom transfer radical polymerization, peroxidase activity as well as working at a biologically relevant pH of 7.4. Depending on the monomer:initiator ratio, spherical, worm-like or vesicular morphologies could be observed, as well as their stability in relevant aqueous solutions like Luria-Bertani broth or RPMI-1640. Differentially sized molecules including enzymes could be encapsulated and functional enzyme cascades could be shown. The ability of GUVs to respond to external stimuli by altering their shape and structure was demonstrated by magnesium-triggered changes in the internal architecture and the conversion of dissolved calcium glycerophosphate to insoluble calcium phosphate by alkaline phosphatase, imitating the production of the mineral matrix of bones by osteoblasts. Encapsulated plasmids and Escherichia coli lysate mimic protein expression, shown by the production of mClover, F-actin and Calcium phosphate. The authors conclude that bioPISA will pave the way for hybrid systems combining synthetic polymers with natural molecules for applications in synthetic biology.Cell-sized asymmetric phospholipid-amphiphilic protein vesicles with growth, fission, and molecule transportation (Suzuki and Kamiya 2023)
The authors generated cell-sized asymmetric phospholipid-amphiphilic protein vesicles composed of a lipid membrane on the outer leaflet and an oleosin membrane on the inner leaflet. Oleosins are plant proteins that stabilize the oil body. Insertion of the outer membrane protein G into the membrane led to nanopore formation and transport of ions and small molecules. Addition of lysophosphatidylcholine micelles facilitated an increase of vesicle size, eventually leading to deformation and fission. The authors conclude that this new vesicle has the potential as a novel cellular model that combines the advantages of lipid and protein leaflets, including the self-replication system, communication of living cells, and complex metabolic pathways. -
2022
Programmable fusion and differentiation of synthetic minimal cells (Gaut et al. 2022) the authors developed a combinatorial genetic circuit assembly technology, where populations of synthetic cells (liposomes) can control individual gene components and can be combined via fusion (“mating”) to construct complex biological pathways (in synthetic cells). Before, the ability to control expression of enough genes to build complex genetic pathways and the ability to mate and differentiate populations into separate lineages were yet elusive in synthetic cells. The synthetic cell mating system of Gaut et al. is based on a programmable, fully orthogonal liposome fusion via surface DNA tags. More precisely, they used liposomes with Escherichia coli cell-free protein expression systems that carry different DNA tags and contain a series of RNA polymerases and recombinases. Liposome fusion is targeted via the DNA tags that have a counterpart on another liposome population and as a result of different fusion events genetic circuits are activated. The authors demonstrated the utility of this technology to create lineages of synthetic cells, differentiating “ancestral pluripotent” populations into independent lineages upon sensing of a small molecule signal (or upon fusion with another population of synthetic cells).
A DNA origami rotary ratchet motor (Pumm et al. 2022) the authors developed a nanoscale rotary motor built from DNA origami that is driven by ratcheting where energy is needed to overcome physical obstacles. The motor has the potential to drive uphill synthesis reactions. Its mechanical capabilities approach those of biological motors such as F1F0-ATPases. After attachment of the motor to a microscope glass coverslip fluorescent dyes at the tips of each rotary arm enable experimental observation of the motion. To reach a biased movement a non-rotating electric alternating current (AC) field was applied. The field causes an alternating ion current flowing through the sample chamber along a fixed axis. Depending on the nature and location of energetic minima in the motor relative to the axis of the electric field, the modulation can produce a kinetic asymmetry per field cycle that causes the motor to move with a preferred rotation direction. The authors successfully encoded the motor in DNA sequences and self-assembled it in reaction mixtures. They showed a rotation with directional bias, where the direction is randomly set. The maximum velocity recorded was comparable to the power of natural molecular machines such as the ATP synthase. The direction of rotation and the effective angular speed of each motor particle could be controlled by the orientation of the AC field axis relative to the motor particles (which are fixed on the substrate).
Implanted synthetic cells trigger tissue angiogenesis through de novo production of recombinant growth factors (Chen et al. 2022) the authors generated synthetic cells (SCs, autonomous protein-manufacturing cell-like particles) that produce proangiogenic factors and are able to trigger the physiological process of neovascularization in mice. The cells could serve as a tool replacing diseased natural cells and addressing medical needs. The SCs were constructed of giant lipid vesicles and were optimized to facilitate enhanced protein production. When introduced with the appropriate genetic code, the SCs synthesized a recombinant human basic fibroblast growth factor (bFGF), reaching expression levels of up to 9 x 106 protein copies per SC. The release of the protein took place via natural membrane permeability. In culture, the SCs induced endothelial cell proliferation, migration, tube formation, and angiogenesis-related intracellular signalling, confirming their proangiogenic activity. Integrating the SCs with bioengineered constructs bearing endothelial cells promoted the remodelling of mature vascular networks, supported by a collagen-IV basement membrane-like matrix. In vivo, prolonged local administration of the SCs in mice triggered the infiltration of blood vessels into implanted Matrigel plugs (mimicking the physiological cell matrix and used to study angiogenesis) without recorded systemic immunogenicity.
Building artificial plant cell wall on lipid bilayer by assembling polysaccharides and engineered proteins (Notova et al. 2022) the authors achieved the assembly of complete plant cell wall mimics by using an engineered chimeric protein designed for bridging pectin to the cellulose/hemicellulose network. Such artificial cell walls can serve as a basis for the development of (experimental) plant cell mechanical models. So far existing models lacked the pectin component. Here, the authors used the carbohydrate-binding module from Ruminococcus flavefaciens engineered for high affinity binding to polygalacturan, a main component of the pectin network, and combined it with the lectin of Ralstonia solanearum, engineered for strong binding of hemicellulose. Thereby a protein glue is created that stabilizes the interaction between hemicellulose and pectin for the assembly of an artificial cell wall. A two-dimensional assembly of an artificial plant cell wall was built first on synthetic polymer and afterwards on supported lipid bilayer.
Living material assembly of bacteriogenic protocells (Xu et al. 2022) the authors demonstrate a new approach to generate artificial cells by using components of living systems as the basis. They organized pieces of broken-down bacteria cells on a synthetic scaffold of a coacervate thereby producing artificial cells with functional and compositional complexity reminiscent of living cells. The work shows that coacervates have a huge potential as platforms to localize and integrate diverse biomaterials, including living cells, to make artificial cells. First, coacervates (membrane-free droplets made of dense liquid phase that form and separate spontaneously from aqueous solutions) to make the synthetic scaffold where generated. The coacervates were formed through the associative interactions between a synthetic polymer and ATP. This platform was then used to capture two types of bacteria (Escherichia coli and Pseudomonas aeruginosa (PAO1 strain)), one on the droplet’s surface and the other type inside the droplets. The rupture of the bacteria cells than led to artificial cells consisting of a coacervate with an outer, bacterium-derived membrane and bacterium derived subcompartments/container comprising active enzymes, a functional protein synthesis machinery, vacuoles and plasmid DNA. The authors demonstrated that the cell core carries out transcription and translation using the bacterial components. They also achieved subcellular organization by using an enzyme to cleave the bacterial DNA into short strands and adding a negatively charged polymer plus histones which caused the DNA to condense into a nucleus-like structure. Furthermore, actin proteins could be introduced and assembled into fibres, providing a rudimentary cytoskeleton. As a step towards self-sustainable energization E. coli cells were implanted as surrogate mitochondria for energization by ATP. This resulted in the artificial cell’s morphing into an amoeba-like shape.
Dendrimersome synthetic cells harbor cell division machinery of bacteria (Wagner et al. 2022) the authors show the reconstitution of a rudimentary/active bacterial divisome in a xenobiotic system based on dendrimersomes. The dendrimersomes were assembled from Janus dendrimers (JD) and are particularly stable compared to other synthetic compartments such as liposomes. The authors used two Janus dendrimers: JDPC contains a zwitterionic phosphocholine headgroup and confers membrane stealth to proteins and JDPG has a phosphoglycerol headgroup for interaction with proteins. The MinCDE protein system, which self-assembles into protein patterns, directs the assembly of FtsZ protein filaments at mid-cell in Escherichia coli to form a contractile division ring. The JDs interact with the Min proteins and this interaction can be varied by the JDPG:JDPC ratio. That way, Min protein patterns in dendrimersomes were regulated from irreversible binding or no attraction to a dynamic pattern formation. The compartmentalised Min proteins showed either pole-to-pole oscillations, pulsing or traveling waves, with the first one being characteristic for living cells. The authors showed that the Min system could direct the dynamic assembly and positioning of FtsZ filaments at the membrane, thus representing the first example of an active cell division machinery incorporated into a fully synthetic vesicle. This modular system could be used to develop functional synthetic cells.
In vitro assembly, positioning and contraction of a division ring in minimal cells (Kohyama et al. 2022) the authors fully reconstituted the cell division machinery of Escherichia coli in lipid vesicles. This presents a major step towards autonomous self-replication in artificial cells/towards developing a synthetic cell. The E. coli divisome consists of the MinCDE protein system guiding assembly and positioning of a presumably contractile ring based on FtsZ and its membrane adaptor FtsA. The authors used two approaches to successfully demonstrate Min wave-assisted FtsZ-ring assembly within lipid vesicles (giant unilamellar vesicles (GUVs), more than ten times the size of bacterial cells): (1) a fully controlled system with purified proteins and (2) a specifically tailored assay with cell-free protein expression in vesicles. In the first assay they could show that crowded environments are essential to form FtsZ-ring structures. The second assay supported a time-sensitive sequential expression of all the five components and showed that the timing of events is crucial. In contrast to the system with purified proteins, the time-controlled series of events of FtsZ self-assembly and MinCDE oscillation resulted in a noticeable shape transformation of the spherical vesicle along with the Min induced centric condensation of an originally isotropic FtsZ-FtsA meshwork.
Bottom-up assembly of synthetic cells with a DNA cytoskeleton (Jahnke et al. 2022) the authors realized the de novo assembly of entirely artificial DNA-based cytoskeletons with programmed multifunctionality inside vesicles and by this showed the potential of DNA nanotechnology to mimic the diverse functions of a cytoskeleton in synthetic cells. More precisely, the authors used giant unilamellar lipid vesicles (GUVs) in which the DNA cytoskeletons are repeatedly and reversibly assembled and disassembled through exposure to light using the cis−trans isomerization of an azobenzene moiety positioned in the DNA tiles. They induced ordered bundling of hundreds of DNA filaments into more rigid structures using molecular crowders and their persistence length is tuned via the choice of crowder. That way, they could achieve the formation of ring-like cortical structures inside GUVs, resembling actin rings that form during cell division. Additionally, the authors show that DNA filaments can be programmably linked to the compartment periphery using cholesterol-tagged DNA as a linker. The linker concentration determines the degree of the cortex-like network formation, and they demonstrated that the DNA cortex-like network can deform GUVs from within. In the future, it is envisaged to equip DNA filaments with molecular motors for intracellular cargo transport, force generation and contractility.
Cell-mimic directional cargo transportation in a visible-light-activated colloidal motor/lipid tube system (Ghellab et al. 2022) the authors designed a system mimicking the subcellular traffic system in living cells that is composed of colloidal motors and self-assembled lipid tubes. The colloidal motors are composed of asymmetrically gold-coated hematite and display light-activated self-propelled motion upon green-light activation when hydrogen peroxide as a fuel is present. The motion is dependent on light intensity and fuel concentration. Importantly, the motors show light-switchable binding with lipid cargoes and attachment to the lipid tubes, whereby the latter act as the motor highways. Upon assembly, the colloidal motor/lipid tube system demonstrates directional delivery of lipid vesicles, emulating intracellular transportation. The lipid tubular track allows light-switchable motion and the transportation and release of cargo thereby resembling the kinesin/microtubule system. This might be a promising platform for various applications, for example in material science or in synthetic cells.
-
2021
Light-powered reactivation of flagella and contraction of microtubule networks toward building an artificial cell (Ahmad et al. 2021): the authors tried to build a self-sustained system with an ATP-driven directed movement. To achieve this, the authors constructed switchable photosynthetic liposomes that contain bacteriorhodopsin and an ATP synthase from Escherichia coli that can convert ADP to ATP upon light illumination. They coupled the module to the functional flagella module isolated from Chlamydomonas reinhardtii and observed the conversion of light into mechanical work. In another approach, the ATP regeneration module was encapsulated with microtubules and kinesin-1 molecular motors and contractions of the filamentous network were observed upon light stimulation. This work is a further step towards advanced synthetic cells which are able to use a broad range of cytoskeleton dependent motor-driven functions.
Programmable microbial ink for 3D printing of living materials produced from genetically engineered protein nanofibers (Duraj-Thatte et al. 2021) the authors developed a microbial ink that is produced by genetically engineered E. coli and can be functionalized. The ink consists of nanofibers made up of bacterial curli fibres. The curli fibres gene CsgA was modified so that the curli proteins are fused at its ends to a knob and a hole protein domain derived from fibrin. These domains allow the self-assembly into nanofibers that are printable into a 3D hydrogel. The hydrogel can be functionalized by incorporating e. g. genetically engineered E. coli that produce an anticancer drug upon induction, sequester the toxic chemical bisphenol A, or regulate their own growth.
Reconstitution of contractile actomyosin rings in vesicles (Litschel et al. 2021) the authors induced the formation of membrane-bound actin rings in giant unilamellar vesicles that resemble the contractile division ring in many cells. The authors encapsulated G-actin with the actin-bundling proteins talin and vinculin and linked the actin filaments to the phospholipid bilayer via biotin-neutravidin bonds. When muscle myosin was included, contractile actomyosin rings were formed that can be seen as a first step towards a minimal divisome for protocells. The rings can direct contractile forces directly to the membrane leading to a shape deformation of the vesicle.
Compacting a synthetic yeast chromosome arm (Luo et al. 2021) the authors have reduced the genome of Saccharomyces cerevisiae by using the loxP-sites integrated into synthetic chromosomes during the synthetic yeast genome project Sc2.0. These sites were integrated to allow for a process called SCRaMbLE (Synthetic Chromosome Rearrangement and Modification by LoxPsym-mediated Evolution) that generates genetically diverse yeast cells via inversion, deletion, duplication, and translocation upon expression of the Cre recombinase. The authors integrated selectable marker genes in the sequence between two loxP-sites, so-called loxP-units (LU), allowing the identification of strains in which an LU is lost. They tested this method using a yeast strain containing the synthetic left arm of chromosome XII (synXIIL) and identified a strain that lost 26 % (45 kbp) of synXIIL. Greater reduction of chromosome synXIIL was difficult to achieve because some nonessential genes were located in the same LU as an essential gene. To overcome this, an episomal gene array carrying essential genes was constructed to compensate for the loss of these genes. That way, some LUs containing essential and non-essential genes could be deleted resulting in a yeast strain in which 58 % (100 kb) of synXIIL was deleted. The reduction of the yeast genome is thought to produce better chassis organisms for the production of metabolites as less energy is consumed for unnecessary processes.
Programmable Aggregation of Artificial Cells with DNA Signals (Qiu et al. 2021) the authors studied programmable communication and coordination between vesicles using DNA strands. They created giant unilamellar and small unilamellar vesicles (GUV and SUV) that interact with each other upon a DNA signal. The GUVs possess pores made from DNA origami that are closed with planar DNA origami caps. The caps can be removed by a toehold-mediated strand displacement opening the pores for the incorporation of an external DNA hairpin. The hairpin DNA can bind both the GUV and the SUV, but only when cleaved by an enzyme encapsulated in the GUV. After cleavage the hairpin diffuses out of the GUV and binds both vesicles so that SUVs aggregate on the GUV. This aggregation can be reversed by a “releaser strand” complementary to the transduced hairpin. The system developed here can be used for cell-cell communication and coordinated cell behaviour in bottom-up synthetic biology.
-
2020
Biner et al. (2020) constructed and characterized energy-regenerating nanovesicles (erNV) mimicking the oxidative phosphorylation of mitochondria to regenerate the pool of adenosine triphosphate (ATP). In order to generate erNVs, a lipid mixture was mixed with a minimal set of respiratory chain relevant units, such as ubiquinone-10 (Q10), ATP synthase complex (Ec-F1F0) and complex I (mito-CI) from common cattle (Bos taurus) and the alternative oxidase (AOX) from Trypanosoma brucei brucei. In theory, the erNVs should be able to use electrons from the oxidization of the substrate nicotinamide adenine dinucleotide (NADH) to NAD+ to generate a electrochemical proton motive force (Δp) and synthesize ATP. Experimental data from different assays confirmed that all components are inserted predominantly in their functional active orientation within the vesicle membrane and that the erNVs are able to produce ATP. Compared to sub-mitochondrial or sub-bacterial vesicles prepared from native mammalian or bacterial membranes, the erNVs are able to maintain a similar and high Δp, meaning only 50 % of the protons are lost due to leakage and ATP can be stably synthesized. This work represents the first example of a semi-synthetic system to drive ATP regeneration by NADH oxygenation. - to the original literature
Buddingh’ et al. (2020) constructed an intercellular allosterically activated communication network between artificial cells. In order to implement the communication platform, two populations of artificial cells (giant unilamellar vesicles) were designed. The “sender” cells respond to an external trigger by producing adenosine 5′-monophosphate (AMP) as a small signalling molecule that is released into the medium. The “receiver” cells recognize and amplify the signal, using the allosteric AMP dependent activation of the glycogen phosphorylase b (GPb). Compared to the background activity in absence of AMP, even low concentrations of AMP lead to a conformational change in GPb in “receiver” cells, resulting in a high rate of glycogen phosphorolysis and an 80-fold increase of nicotinamide adenine dinucleotide (NADH) production. This is the first allosterically activated communication network, which not only fully relies on the protein machinery and small molecules as chemical information agents but is also able to facilitate the propagation of signals over long distances within the artificial cell consortia. - to the original literature
Fan et al. (2020) generated a simplified synthetic biology chassis by creating chromosome-free simple cells (SimCells) from Escherichia coli, Pseudomonas putida and Ralstonia eutropha. To remove the native chromosomes, the heterologous I-CeuI endonuclease and the degradative activity of endogenous nucleases were used to induce several double strand breaks within the chromosomes and the complete breakdown of the genome. The generated SimCells remained functional and stable. In fact, SimCells were able to express introduced synthetic pathways for at least ten days. In a proof of principle experiment, SimCells, synthesizing anti-cancer drugs (e.g. catechol) could be used to significantly decrease cancer cell viability in cell culture after incubation with these SimCells. SimCells represent a synthetic tool that can be programmed to manufacture and deliver medicinal products safely without interference with the host genome. - to the original literature
Yang et al. (2020) developed protocells as minimalistic communication models for cell-cell communication studies based on the model published in 2019 by Joesaar et al.. The authors built two different proteinosome-based semipermeable protocells containing biotinylated DNA complexes capable of either sending or receiving. Upon laser irradiation a photocleavable nitrobenzyl linker is cleaved off and releases a DNA strand. This DNA strand can reach the receiver cells where it results in a fluorescent response. By changing the experimental conditions, the authors showed that the signal range is determined by several factors such as density and permeability of receivers, extracellular signal degradation, signal consumption and catalytic regeneration. They also created receiver cell with an AND receiver that was activated spatiotemporally by two sender cells.- to the original literature
-
2019
Berhanu et al. (2019) developed an artificial cell system with a self-supplying energy production for protein synthesis. They created giant unilamellar vesicles with an artificial organelle that contained the membrane proteins bacteriorhodopsin and F-type-ATP-synthase. The bacteriorhodopsin pumps protons into the organelle which can be used by the ATP synthase to produce ATP. When a modified transcription-translation system was added the produced ATP can be used as a substrate for messenger RNA (mRNA), for the phosphorylation of GDP and for the aminoacylation of tRNA. As a result, bacteriorhodopsin and subunits of the ATP synthase could be synthetized de novo in this system thereby increasing photosynthetic ATP production in the artificial organelles. - to the original literature
Diederichs et al. (2019) - The authors developed molecules for the formation of nanopores with a diameter of 20.5 nm and an average height of 31.5 nm, through which folded proteins such as fluorescent proteins can also be transported. The nanopores comprise squarely arranged DNA duplexes that are provided with cholesterol-lipid anchors for insertion in the membrane. There thus result 50 nm2-sized pores that could be inserted into artificial cells. - to the original literature
Ghosh et al. (2019) - A diffuse movement during substrate turnover can be observed with many enzymes, increasing even more with an increase in the substrate concentration. Making use of this fact, Ghosh et al. produced phospholipid vesicles that contained Na+/K+-ATPase as a transmembrane molecule. These vesicles were then tested in culture under physiological conditions and showed directed movement, followed by randomised reorientation. These vesicles represent a first step in the direction of autonomous nanovesicles and can be used e.g. as transport vesicles for biomolecules. - to the original literature
For the further development of synthetic cells, mechanosensitive mechanisms for various uses were implemented by two different research groups, as follows:
Garamella et al. (2019) constructed protocells that react to environmental influences and can independently counteract a stimulus. For this, synthetic cells with a phospholipid membrane were produced that are mechanosensitive through the incorporation of MscL ion channels. The intracellular space of the synthetic cells contains plasmids for expression of a bacterial cytoskeletal protein (MreB) and also an in vitro transcription- and translation system. By changing the outer environmental conditions toward a hypoosmolar exterior there arises a mechanical stress to the membrane and thus pore formation through the MscL. A regulator molecule located in the extracellular medium subsequently flows in, which makes the expression of the MreB possible. MreB then migrates to the membrane and increases the mechanical robustness there. An adaptive synthetic cell system could herewith be created that showed resistance to osmolar stress. - to the original literature
Hindley et al. (2019) established a mechanosensitive signal path based on the sPLA2-membrane-MscL network by Charalambous et al. (2012). The calcium-dependent soluble phospholipase A2 (sPLA2) thereby served as the catalyst to synthesize lysophosphatidylcholine, which is incorporated into the membrane of artificial cells. This in turn leads to a change in the membrane mechanics and to the opening of the ion channel MscL. Building on this, Hindley et al. created artificial cells that contained mechanosensitive vesicles with the fluorescent dye calcein as well as inactivated sPLA2. The subsequent extracellular addition of alpha-haemolysin led to the formation of pores in the outer membrane, then to calcium influx and to activation of sPLA2. The changed mechanics of the vesicle membrane and the resulting opening of the MscL was verified by means of increased calcein fluorescence. A controlled calcium inflow for future signal transduction in artificial cells was thus established. - to the original literature
Joesaar et al. (2019) developed consortia of protocells that are able to correspond with each other through orthogonal channels. These protocells possess a proteinosome that is permeable for short (< 100 bases) single-stranded DNA (ssDNA) and that protects the intra-protocellular DNA from degradation in the culture medium. The protocells are able to communicate via those ssDNA, by using the following scheme: cell one releases an input-ssDNA, this input-ssDNA enters the next cells and replaces another ssDNA with a short mismatch. This second ssDNA is subsequently released and can diffuse into another cell. This system demonstrates a bidirectional communication between different protocell-populations. - to the original literature
Venetz et al. (2019) synthesized a minimal bacterial genome of the model organism Caulobacter ethensis. Already known essential genes were newly assembled with the help of an algorithm, while keeping the original gene organization and orientation. In addition, the genome was streamlined for synthesis. For this purpose, 10,172 base substitutions were introduced to eliminate 1233 repeats, 93 homopolymeric regions, 4342 regions of high GC content and 1045 endonuclease restriction sites. On top, another 123,141 base substitutions were introduced into protein coding regions. The newly synthesized genome (C. eth-2.0) was condensed to 785 kb with 676 protein-coding, 54 non-coding and 1015 intergenic sequences. 56.1 % of all codons were replaced by synonymous codons to eliminate 87.4 % of alternative ORFs, 95.3 % of predicted internal transcription initiation sites and 76.7 % of ribosome stalling motives. 81.5 % of C. eth-2.0 genes demonstrated functionality, indicating that the primary sequence of the mRNA, the secondary structure, or a codon´s context in the genome seem to have no significant impact on the biological function of the coded protein. - to the original literature
-
2018
Niederholtmeyer et al. (2018) manufactured a synthetic protocell with a porous membrane and a primitive “nucleus”. This “nucleus” is a hydrogel compartment built from clay minerals and contains the cellular DNA. The synthetic cells can take up all components of a cell-free transcription and translation system and express the DNA from their “nucleus”. The expressed proteins are able to diffuse to neighboring synthetic cells as a form of cell-cell communication, enabling an artificial quorum sensing. - to the original literatur
Shao et al. (2018) merged all 16 Chromosomes of S. cerevisiae into one so-called super-chromosome, in which all non-essential, telomeres, centromeres as well as 19 repetitive sequences were removed by using CRISPR-Cas9 and the residual chromosome-parts were fused. In a similar approach, Lou et al. (2018) were able to reduce the number of yeast chromosomes to two. These experiments were based on the observation that the number of chromosomes in eukaryotes seems to be rather arbitrary and independent of the quantity of genetic information.
Indeed, the reduction of chromosomes had only little impact on most of the yeast´s characteristics. The cells showed a similar phenotype and only modest transcriptome changes. Growth was slowed-down only in the yeast with a single chromosome. Both yeasts showed a significant reduction in the formation of ascospores as part of sexual reproduction. By reorganizing the genome the new organism was unable to cross-breed with wild-type yeast and is thus reproductively isolated. Hereby, the classic criterion for the assignment to different biological species is met. - to the original literature: Shao et al. (2018), Luo et al. (2018)
Xenobiology
-
2023
Expanding the substrate scope of pyrrolysyl-transfer RNA synthetase enzymes to include non-α-amino acids in vitro and in vivo (Fricke et al. 2023)
The authors describe the first aminoacyl-tRNA synthetase enzyme that accepts α-thio acids and α-carboxy acids that could support carbon-carbon bond formation within the ribosome, increasing the number of non-L-α-amino acids that can be incorporated into proteins in vivo. The structure of Methanomethylophilus alvus pyrrolysyl-tRNA synthetase (MaPylRS) variant MaFRSA was investigated, showing the ability to support biosynthesis of proteins with internal aromatic α-hydroxy acid monomers. The authors conclude that combined with synthetic genomes, ribosomes capable of carbon-carbon bond formation would set the stage for template-driven biosynthesis of unique hybrid biomaterials and sequence-defined polyketide-peptide oligomers. -
2022
For the year 2022, no publications from the field of xenobiology were selected for publication on the ZKBS homepage.
-
2021
Multiplex suppression of four quadruplet codons via tRNA directed evolution (DeBenedictis et al. 2021) the authors used directed evolution to establish a small set of quadruplet tRNAs (qtRNAs) that incorporate amino acids under multiplexing conditions. By introducing a quadruplet codon as a reporter into the β-galactosidase gene, codon-anticodon interactions were first validated in E. coli. With this strategy, 24 qtRNAs were identified that would decode the introduced codon and, as a result, charge a defined amino acid onto the protein. The identified qtRNAs were further evolved by directed evolution in phages. The phages could only propagate if a certain quadruplet codon was decoded. The authors showed the translation of six UAGA-codons in a row as well as the translation of four different quadruplet codons in the same protein. The work is a further step towards an exclusively quadruplet codon translation system.
Sense codon reassignment enables viral resistance and encoded polymer synthesis (Robertson et al. 2021) the authors liberated sense codons to incorporate multiple distinct non canonical amino acids (ncAAs). Using an engineered tRNA-aminoacyl-tRNA synthetase (aaRS) pair, ncAAs can be incorporated as a reaction to a specific codon. The authors used the E. coli strain Syn61 developed by Fredens et al., in which two serine codons (TCG and TCA) as well as the stop codon UAG were exchanged for synonymous codons and evolved the strain so that the tRNAs and release factor 1, which decodes TCG, TCA, and TAG, were deleted. The deletion of these tRNAs and the release factor made the cells resistant to phages that harbour the three codons in their genome. The codons were also reassigned for the incorporation of three distinct ncAAs into a protein. Incorporation of ncAAs into proteins can be used for the development of new biomaterials or biotherapeutics.
-
2020
Chen et al. (2020) created an Escherichia coli strain, which autonomously biosynthesizes 5-hydroxytryptophan (5HTP) as a 21st noncanonical amino acid (ncAA) and incorporates it into proteins. This represents a tool for the evolution of novel proteins containing ncAAs and may be used to generate new therapeutic proteins. In order to use 5HTP as a ncAA in prokaryotes the hydroxylation machinery of tryptophan (the substrate for 5HTP-generation) had to be introduced into E. coli. In a first step, a plasmid harbouring a specific hydroxylase (e.g. XcP4H from Xanthomonas campestris), an artificial recycling pathway for the hydroxylase co-factor tetrahydromonapterin (MH4), and an expression cassette for the green fluorescence protein (GFP) containing one amber stop codon at Tyr151 was generated. A second plasmid which contains a modified tryptophanyl-tRNA synthetase (ScTrpRS)/tRNATrp from Saccharomyces cerevisiae provides the bioorthogonal machinery for incorporating 5HTP at the amber codon. Both plasmids were transferred into E. coli which led to a hydrolase- and MH4 recycle genes-dependent expression of GFP with an efficient and specific 5HTP incorporation. As a proof concept, Chen et al. used E. coli to express the 5HTP containing single-chain variable fragment (scFv) of the anti-human epidermal growth factor 2 (HER2). - to the original literature
Fischer et al. (2020) identified further unnatural codons that could be inserted into the semi synthetic organism (SSO) generated by Zhang et al. (2017). Since the SSO (Escherichia coli) is able to replicate and transcribe the unnatural base pair (UBP) NaM and TPT3 in different sequence contexts, Fisher et al. used this UBP to systematically analyse unnatural codons. Inserting the NaM/TPT3 base pair has the potential to create new codons. In order to test these unnatural codons, GFP-expressing plasmids with NaM or TPT3 at the first, second or third position of codon 151 and tRNAs with cognate unnatural anticodons to enable the incorporation of non-canonical amino acids (ncAAs) in the protein were constructed. Plasmids were then used to transform E. coli. With the help of GFP fluorescence, nine functional codon-anticodon pairs were identified. All of them are stably encoded in the DNA and are transcribed into mRNA and bound by their specific tRNA, thereby efficiently mediating the decoding at the ribosome. Moreover, three of these codon-anticodon pairs were further characterized in E. coli by inserting them simultaneously into the GFP gene along with the corresponding anticodons that were inserted into three tRNAs capable of charging different ncAAs. The three codons were orthogonal to each other and could be simultaneously decoded within the SSO, thereby enabling the cell for the first time to decode 67 codons. - to the original literature
Flamme et al. (2020) have enzymatically created unnatural base pairs (UBPs) on the basis of metal ions. These metal base pairs are orthogonal to the natural Watson-Crick base pairs and integrate easily into the DNA´s structure. The incorporation of metal base pairs expands the chemical diversity of DNA and RNA and can be used for novel nanomaterials.
The authors used two synthetic analogues for the enzymatic synthesis of a silver-mediated UBP: the imidazole containing nucleoside dIMC and the 6-pyridylpurine nucleoside dPurP. This silver-mediated UBP (dIMC - AgI - dPurP) was then incorporated into a DNA duplex using a two-step synthesis protocol. - to the original literatureLee et al. (2020) addressed how the incorporation of non-canonical, backbone extended amino acids (amino acis analogs) into natural polypeptides can be improved and present a further step towards synthesizing non-canonical sequence-defined polymers. The charging of a tRNA with a long chain carbon structure is currently difficult and was studied in the flexizyme system (Fx, an aminoacyl tRNA synthetase-like ribozyme). It was shown that charging of amino acid analogs with linear carbon chains was hindered by the formation of lactams. To address this, intramolecular lactam formation was prevented by steric restriction of the amino and activated ester functionalities. This architecture used a rigid spacer like vinyl or a cyclic centre structure in the central region of the amino acid and increased the overall charging efficiency of the Fx-system. To transfer these findings to a more natural RNA translation, the Fx-charged tRNAs were applied to a cell-free protein synthesis system to incorporate the amino acid analogs at the C- and N-terminus during protein synthesis. It was shown that every substrate that could be loaded onto tRNAs was successfully incorporated into a peptide by wild-type as well as by engineered ribosomes. - to the original literature
Yang et al. (2020) have expanded the studies of Eremeeva et al. (2017), who showed the amplification of DNA with four non-canonical base pairs (DZA). These chemical modifications can enhance the stability and versatility of nucleic acids for new applications, e. g. in biomedical research. The authors tested fully base-modified RNA (RZA) in replication, transcription and reverse transcription and established a flow of genetic information between natural and unnatural genetic information. In vitro, the PCR-synthesized DZA could be transcribed into RZA or into unmodified RNA, and reverse transcribed into DNA or DZA. In vivo, DZA was transcribed and translated into the red fluorescent protein mTangerine in Escherichia coli. - to the original literature
-
2019
Calles et al. (2019) produced a genetic fail safe code that can be read by the natural translation machinery and features a protective effect against spontaneous mutations. Each amino acid is thereby coded by a single codon, all other codons for this amino acid were non-translatable "null-codons". Ideally, the mutation of one of these sense codons should not lead to another sense codon. This is only possible with a 3-base code if only 16 amino acids are coded. However, if a 4-base code is used, all 20 amino acids and, additionally, non-natural amino acids can be coded without a mutation resulting in another sense codon. In a first application of the fail safe code an in vitro transcription and translation system was used. In this system all natural tRNAs were depleted and replaced by 20 tRNAs, that recognized the 20 codons of the fail safe code. No tRNAs were thus available that could recognize the "null-codon“. -to the original literature
Hoshika et al. (2019) expanded the genetic code by adding four synthetic nucleotides und thus created an 8-letter code (Hachimoji). The synthetic 8-letter DNA is largely similar to the natural DNA: it forms a double helix in which interaction occurs via hydrogen bonds and can be transcribed into RNA. However, there are no organisms so far that use and stably pass on the 8-letter code to the next generation. - to the original literature
Kneuttinger et al. (2019) - The authors inserted non-natural amino acids (nnamino acids) in the bienzyme imidazole-glycerol phosphate synthase to achieve the activation of the enzyme by means of light. In the original mechanism the activation of the HisH subunit took place exclusively through the binding of a substrate to the HisF subunit. By incorporating the three light-sensitive nnamino acids phenylalanine-4'-azobenzene, o-nitropiperonyl-O-tyrosine and methyl-o-nitropiperonyllysine in the substrate binding enzyme subunit a light-inducible and reversible increase in activation of the HisH activity was achieved, so that spatial and temporal control of enzyme activity is made possible through light. - to the original literature
Koh et al. (2019) produced auxotrophic Escherichia coli bacteria in which the β-subunit of DNA polymerase III-holoenzyme at position 273 contained synthetic p-benzoylphenyl-alanine (pBzF) instead of a leucine and that therefore could grow only in the presence of pBzF in the medium. The β-subunit that is also described as a sliding clamp is strictly conserved in Gram-negative bacteria, so that the auxotrophic strain features a non-detectable escape frequency. The described auxotrophy can additionally be easily transferred to other bacteria such as Pseudomonas aeruginosa or Acinetobacter baumanii in order to produce conditionally viable living vaccine against these hospital germs. - to the original literature
Reinkemeier et al. (2019) work on the orthogonal translation of the amber stop codon (TAG). To liberate this codon for the incorporation of a non-canonical amino acid into a protein, an orthogonal tRNA/tRNA synthetase pair is required. The tRNA/tRNA synthetase pair and a mRNA, whose amber stop codon was to be recoded, were brought into close proximity inside an artificial organelle. For this purpose, the tRNA/tRNA synthase pair and an RNA-binding domain were fused to a so-called assembler protein. Proteins used as assembler proteins may be proteins that undergo a phase separation in the cell or kinesins that enrich at the microtubule plus ends. The tRNA/tRNA synthase pair as well as an mRNA with a recognition sequence for the RNA-binding domain are enclosed in close proximity, enabling the recoding of the stop codon only in selected RNAs. - to the original literature
Swenson et al. (2019) developed a synthetic, bilingual biopolymer that unites the properties of an amino acid sequence and a nucleotide sequence in a single peptide nucleic acid (PNA) scaffold. The PNA strand thereby has amino acid-like side chains that, based on their arrangement, feature a similar aggregation or folding behaviour as a peptide. Additionally, the PNA strand however also carries a nucleotide in a specific sequence succession that can be bound by complementary DNA or RNA. As an application, the authors showed that PNA strands with hydrophobic and hydrophilic amino acid side chains come together into micelles in an aqueous milieu. The addition of RNA complementary to a nucleic acid sequence contained in the PNA strand caused the micelles to become disordered again. The PNAs should also be used to mediate a precise interaction between various target proteins and nucleic acids. - to the original literature
Methods with impact on Synthetic Biology
-
2023
A split ribozyme that links detection of a native RNA to orthogonal protein outputs (Gambill et al. 2023)
The authors developed a strategy for the detection of specific RNA molecules through the use of ribozymes. In this plug-and-play strategy, called Ribozyme-ENabled Detection of RNA (RENDR), a cellular RNA input activates a splicing reaction, which in turn generates an mRNA that codes for any reporter protein (e. g. green fluorescent protein, GFP). The strategy is based on a ribozyme that has been synthetically cleaved into two non-functional fragments, to each of which RNA guide sequences destined to interact with the RNA input are attached. In the presence of the RNA input sequence, the two transcribed ribozyme fragments are colocalised and form a functional ribozyme complex, which splices the target mRNA leading to the expression of the functional reporter protein.
A crucial step in the development of the RENDR platform was the identification of functional split sites in the structure of the splicing ribozyme of Tetrahymena thermophila. For this purpose, a high-throughput laboratory evolution approach was used in which in vitro transposon mutagenesis was coupled with fluorescence-activated cell sorting. Different split sites were identified that enable high sensitivity and dynamics in RNA recognition.
In addition, the authors also developed further RENDR variants that allow for the facile exchange of different genes encoding protein outputs (colorimetric, gaseous, and regulatory outputs) and demonstrate the transferability to different Gram-negative bacteria (Escherichia coli, Shewanella oneidensis and Vibrio natriegens). Finally, the production of pigment-producing enzymes in the presence of antibiotic resistance genes in E. coli was demonstrated as a proof of application, providing a cost-effective and simple approach for the detection of antibiotic-resistant microbes.A polycistronic system for multiplexed and precalibrated expression of multigene pathways in fungi (Yue et al. 2023)
The authors developed a strategy for the assembly of multigene pathways with the required expression level of each gene referred to as HACKing (Highly efficient and Accessible system by CracKing genes into the genome). The strategy is based on the integration of a 9 bp nucleotide sequence (short intergenic sequence 6 (IGG6)) that enables efficient polycistronic gene expression in yeasts and filamentous fungi, coupled with multiplexed CRISPR/Cas9-based genome editing. Results suggest that IGG6 mediates the re-initiation of translation, allowing separate genes to be joined into a functional operon. With HACKing, the expression level of individual enzymes can be pre-calibrated by linking their translation to that of host proteins whose abundance has been pre-determined under the desired fermentation conditions. The HACKing strategy was validated with the construction of biosynthetic pathways of endogenous or heterologous terpenoid products in Saccharomyces cerevisiae. Due to its predictability, simplicity, scalability and speed, HACKing can be utilised in fungal metabolic pathway engineering for valuable metabolites.CRAPS: Chromosomal-Repair-assisted Pathway shuffling in yeast (Dykstra et al. 2023)
A fundamental challenge of metabolic engineering involves assembling and screening vast combinations of orthologous enzymes across a multistep biochemical pathway. Current pathway assembly workflows involve combining genetic parts ex vivo and assembling one pathway configuration per tube or well. Here, the authors present CRAPS (Chromosomal-Repair-Assisted Pathway Shuffling), an in vivo pathway engineering technique that enables the self-assembly of one pathway configuration per cell in a one-step transformation. CRAPS leverages the yeast chromosomal repair (CR) pathway and utilizes a pool of inactive, chromosomally integrated orthologous gene variants corresponding to a target multistep pathway. The CRAPS pathway consists of gene-less expression cassettes, each possessing a unique synthetic Cas9 target site. The gene variants possess partial homology to the flanking promoter and terminator elements and are inactive prior to CR repair. Supplying gRNAs to the CRAPS host introduces a double strand break at each expression locus, activating the CR pathway and placing one gene ortholog under transcriptional control of its designated promoter. Thus, one gene variant per pathway step is expressed, resulting in a unique pathway configuration in each cell and thus colony. The authors deployed CRAPS to build more than 1000 theoretical combinations of a four-step carotenoid biosynthesis network. Sampling the CRAPS pathway space yielded strains with distinct colour phenotypes and carotenoid product profiles.A multiplexed bacterial two-hybrid for rapid characterization of protein–protein interactions and iterative protein design (Boldridge et al. 2023)
The authors developed a method for identifying and designing orthogonal coiled-coil proteins. This strategy involves the development of a next-generation bacterial two-hybrid (NGB2H) method, a significantly modified version of the Bordetella pertussis adenylate cyclase two-hybrid system, which enables multiplex characterization of protein-protein interactions (PPIs). The system uses a barcode approach and allows comprehensive analysis without the need to test all possible pairings manually. Ultimately, large quantities of orthogonal synthetic coiled-coils were designed, created, and tested. The large dataset was used to train a more accurate coiled-coil scoring algorithm (iCipa). This was done iteratively, increasing the size of the synthetically designed libraries from 256 interactions to more than 18,000 interactions. Based on this, the authors have identified what they believe to be the largest set of orthogonal coiled-coils to date, with fifteen on-target interactions. The approach offers a powerful tool for designing orthogonal PPIs.Illuminating protein space with a programmable generative model (Ingraham et al. 2023)
Tthe authors present Chroma, a generative model for proteins and protein complexes, capable of directly generating new protein structures and sequences. Chroma is programmable and can create proteins with a variety of user-defined properties, such as the distance and contact between amino acid residues, specific protein regions and structures, as well as other features defined by classifiers. Chroma can generate proteins with arbitrary and complex shapes and has even begun to demonstrate the ability to accept descriptions of desired properties as free text. It is capable of generating extremely large proteins and protein complexes (with more than 3,000 amino acid residues) on a commodity graphics processor in a few minutes.
The authors selected and experimentally tested some of the proteins generated by Chroma. The experimental validation of 310 proteins showed that Chroma has learned a sufficiently accurate distribution, so that the designed proteins are expressed, fold correctly, have favourable biophysical properties, and often match the intended structures. Additionally, the crystal structures of two designed proteins were determined, showing an atomic-level match with the Chroma samples. -
2022
4-bit adhesion logic enables universal multicellular interface patterning (Kim et al. 2022) the authors have created multicellular interface patterns with swarming bacteria that harbour a cell-cell adhesion logic. They engineered Escherichia coli colonies that express synthetic adhesins derived from nanobodies and their complementary antigens. The bacterial colonies were then seeded a few millimetres apart on soft agar and allowed to grow. The authors observed the formation of interfaces where matching nanobody and antigen met and developed a model of bacterial swarming. The model calculations such as influencing geometric interface properties by tuning seeding conditions were confirmed experimentally. By combining four adhesins (four bits) in two adhesion pairs, any arbitrary tessellation and straight-interface pattern in 2D could be created with the aid of an algorithm. This bacterial adhesion logic could be used, for example, to create human-readable molecular diagnostic displays.
The automated Galaxy-SynBioCAD pipeline for synthetic biology design and engineering (Herisson et al. 2022) the authors introduce the open access Galaxy-SynBioCAD portal, a set of tools for synthetic biology, metabolic engineering and industrial biotechnology. The tools and workflows currently shared on the Galaxy-SynBioCAD portal enable the users to design different metabolic routes and build libraries of strains for the production of a compound of interest. To predict if a given pathway is a valid one or not, a pathway scoring is performed via machine learning using a training set of pathways extracted from literature and pathways validated by pathway engineering experts. The portal is a growing community effort where developers can add new tools and users can evaluate the tools´ performing design for their specific projects. As proof of concept a library of 88 E. coli lycopene-producing strains was designed and engineered. Within this library three enzymes from Pantoea ananas (CrtE, CrtB and CrtI) were assembled in varying gene order in an operon with six different ribosome-binding-sites and two different promoters.
basicsynbio and the BASIC SEVA collection: software and vectors for an established DNA assembly method (Haines et al. 2022) the authors present basicsynbio, an open-source software, that allows users to easily and robustly design a large repertoire of assemblies, enabling applications in synthetic biology and the life sciences. basicsynbio is based on a standardized DNA assembly method developed in 2015 by Storch et al. called Biopart Assembly Standard for Idempotent Cloning (BASIC) DNA assembly. This method utilizes modular parts and linkers as functional units and can assemble up to 14 of these units per round with > 90% accuracy. The method was successfully applied to several areas of research including combinatorial pathway engineering, synthetic operon and small non-coding RNA circuit design, combinatorial guide RNA expression for gene editing, ribosome binding site tuning and fusion protein engineering. The basicsynbio design software is accessible as web app or as python package. Users can access commonly used parts and linkers, robustly design new parts, linkers, and assemblies while exporting sequence data. The exported data can easily be parsed into custom workflows, enabling the automation of BASIC DNA assembly on further liquid-handling platforms and the generation of instructions for manual workflows.
To demonstrate the functionality of basicsynbio, 30 vectors were assembled using modules from the Standard European Vector Architecture (SEVA) database. Each vector contains a specific combination of antibiotic resistance marker and origin of replication.Cross-kingdom expression of synthetic genetic elements promotes discovery of metabolites in the human microbiome (Patel et al. 2022) the authors developed a computational and experimental strategy for the redesign, expression, mobilization and characterization of multigene biological pathways in prokaryotes and eukaryotes. Studying biosynthetic gene clusters (BGCs) in their native context has been hampered by the inability to grow microorganisms outside of their native environments. When cultivation is possible, many BGCs are silenced under laboratory conditions. The new strategy allows for the decoupling of BGCs from native levels of regulation, thereby enabling the discovery of a variety of natural products and biosynthetic pathways.
As a first step in this approach yet uncharacterized BGCs are computationally redesigned into synthetic genetic elements (SGEs) and functionalized for expression across diverse hosts. The computer-aided design of SGEs includes the redesign of each coding sequence by implementing N-terminal codon bias, which creates versatile 5´ hybrid untranslated regions (UTRs), and screening to avoid internal start and termination signals. Furthermore, hybrid eukaryotic and prokaryotic regulatory elements are integrated for cross-kingdom expression. This includes the design of a library of synthetic yeast promoters and an inducible T7 RNA polymerase expression circuit. Finally, chromosomal-integrated landing pads for SGE mobilization were developed for the stable transfer of SGEs across diverse hosts.
To functionally apply this system for natural product discovery the authors targeted an uncharacterized BGC that had previously been computationally predicted from the genome of Lactobacillus iners. A new class of nucleotide metabolites, called tyrocitabines, was discovered.Three-dimensional structure-guided evolution of a ribosome with tethered subunits (Kim et al. 2022) the authors present a directed evolution approach that they used to improve previously designed tethered ribosome systems (Ribo-T) (Carlson et al., 2019). In these tethered ribosome systems, the 16S and 23S rRNAs are joined together to form a single chimeric molecule. The single-subunit ribosomes are able to incorporate unnatural amino acids and synthesize protein sequences that are inaccessible to the natural ribosome. So far the potential of Ribo-T systems was limited by their low activity. Directed evolution is a possible way to improve Ribo-T. However, the complexity of macromolecular machines such as the ribosome complicates genetic manipulation. Macromolecular machines have complex tertiary structures with functionally important residues that are far apart in primary sequence but proximal in three-dimensional space. The authors present Evolink (evolution and linkage), a next-generation library construction approach, which brings together separated regions of interest for a continuous next generation sequencing (NGS) read in a three-step process (PCR, ligation and a second PCR) by using basic and low-cost molecular biology methods. In this work two regions of interest within Ribo-T version 2 (Ribo-T v2) were targeted by directed evolution using a degenerate library of 5-15 nt. Afterwards the regions were connected to enable NGS readout. Central to the efforts was the iterative application of design-build-test-analyse (DBTA) cycles, where multiple libraries can be tested, each library building on results and analysis of previous ones. Finally, Ribo-T v3 was developed with a 58 % increased activity in orthogonal protein translation and a 97 % improvement in doubling times in E. coli SQ171 cells compared to the previously developed tethered ribosome (Ribo-T v2).
In general, the Evolink approach may enable enhanced engineering of macromolecular machines for new and improved functions for synthetic biology.A versatile active learning workflow for optimization of genetic and metabolic networks (Pandi et al. 2022) the authors present METIS (Machine-learning guided Experimental Trials for Improvement of Systems), a modular and versatile active machine learning workflow for data-driven optimization of biological targets with minimal experiments. METIS was developed to overcome the gap between machine learning algorithms and their application for biological systems. Therefore, METIS was created for experimentalists with no experience in programming. METIS runs on Google Colab, a free online platform to write and execute Python codes.
The versatility of METIS was proven on various biological systems. For instance, by identifying a major bottleneck the activity of a recently reported cell-free in vitro gene circuit (LacI-based multi-level controller; Greco et al., 2021) was improved by two orders of magnitude. Also an in vitro transcription-translation system of Escherichia coli (Borkowski et al., 2020) was optimized showing a ten-fold increase in protein production. Finally, METIS was used to improve a complex metabolic network, the so-called crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA (CETCH) cycle (Schwander et al., 2016), a CO2 fixation cycle comprising 17 different enzymes plus 10 cofactors, which is more efficient than natural photosynthesis. For the CETCH cycle 1025 different conditions were explored and 1000 experimental conditions performed. This resulted in a ten-fold improved productivity, representing the most efficient CO2-fixing in vitro system described to date.
Overall, METIS has the ability for the optimization of various complex biological networks with minimal experimental efforts, providing multiple opportunities for the study and engineering of different biological systems in the future.Machine learning-aided engineering of hydrolases for PET depolymerization (Lu et al. 2022) the authors engineered a robust and active polyethylene terephthalate (PET) hydrolase (PETase), an enzyme capable of depolymerization of PET into monomers that can be used in a closed-loop PET recycling process to resynthesize PET. The enzyme was called FAST-PETase (functional, active, stable and tolerant PETase) and designed using a structure-based, machine learning algorithm. FAST-PETase contains five mutations (N233K, R224Q, S121E, D186H and R280A) compared to Ideonella sakaiensis wild-type PETase and shows superior PET-hydrolytic activity between 30 and 50 °C and a range of pH levels. The new enzyme could provide a possible method for the industrial enzymatic degradation of plastic waste.
-
2021
Division and regrowth of phase-separated giant unilamellar vesicles (Dreher et al. 2021) the authors established a fully controlled division mechanism for giant unilamellar vesicles (GUV). The authors used phase separation to construct GUVs with a lipid disordered and a lipid ordered phase. When the osmolarity in the surrounding medium was increased, the GUV´s volume decreased by water efflux and two smaller vesicles were formed. The technique was also used to construct light-inducible vesicle division. To this end, bis-(5-carboxymethoxy-2-nitrobenzyl)-ether (CMNB)-caged fluorescein was added to the GUV containing medium. Upon illumination this initially non-fluorescent compound is split up into three components, so that osmolarity is increased and the GUVs divide. The authors also studied vesicle growth through fusion of lipid disordered and lipid ordered vesicles and showed a fusion with a rather low frequency. The fusion frequency can be increased by using CaCl2 or zipper-like DNA-based mimics of SNARE proteins that bring the vesicles into close proximity to each other and drive fusion. Taken together, this method enables a controllable GUV-division into two second-generation compartments and represents another step towards synthetic cell division.
Manufacture of multi-layered artificial cell membranes through sequential bilayer deposition on emulsion templates (Ip et al. 2021) the authors developed an emulsion-based method to generate multi-layered giant vesicles. To generate these multi-layered vesicles, water-in-oil droplets were used as a template and sequentially surrounded with lipid monolayers. The innermost monolayer droplet was formed by incubating aqueous emulsion droplets in an oil solution. Additional monolayers were added by driving the initial droplet in a centrifuge through several monolayer-stabilized oil/water interfaces with alternating hydrophobic/hydrophilic outside orientation. The generated multi(bi)layer vesicles ranged from 0.5 – 50 µm in radius and were mechanically stable. Successful layering was controlled by adding different fluorescent lipids (Rh-PE, NBD-PE, Cy-5-PE) to each double layer. In addition, sodium dithionite, a membrane impermeable NBD quencher, was added to the vesicle medium. Due to lack in quenching the authors could demonstrate that the inner membranes, containing NBD-PE, are protected by the additionally applied outer membranes, thus verifying a successful and stable layering. The membrane mechanics, like bending rigidity, were tested by thermal fluctuation of the vesicles, detected via phase-contrast microscopy. As a result, the increased number of layers led to an increase in bending rigidity, confirming that the multilayers are mechanically coupled. This method may offer a potential solution for incorporating a broad range of functional modules into synthetic vesicles.
-
2020
Li et al. (2020) established a scalable magnetic assembly of cell-mimicking giant unilamellar vesicle (GUVs) colonies. In order to develop a magnetic field-based colony formation of GUVs, a stainless steel-mesh with a microwell pattern in a paramagnetic medium was loaded with GUVs and placed between two face-to-face magnets. The GUVs were magnetically gathered to form colonies and furthermore exhibited a higher stability against osmotic imbalances compared to suspensions with individual GUVs. To mimic the structural complexity of biological tissue with different cell types, two types of GUVs with different compositions were generated and magnetically manipulated. Depending on the direction of the magnetic field, the different GUVs could be sorted separately in different directions, forming layers or a serial distribution within the microwell. In a proof of principle experiment intended to mimic a spatialized biochemical reaction among organelles, two different GUVs were generated and magnetically separated. GUVs in colony 1 possessed pores and were formed in the presence of glucose oxidase, while GUVs in colony 2 were formed together with horseradish peroxidase. When glucose was added to the medium, it entered GUVs of colony 1 through the pores, H2O2 was produced and diffused into the GUVs of colony 2, where the horseradish peroxidase catalyzed the production of a red fluorophore. Since the red fluorophore could only be detected in colony 2, the system’s ability to rudimentarily mimic the capacity of natural tissue in compartmentalization and spatialization of biochemical reactions was shown. This work could enable the creation and study of tissue like structures and represents an important step towards creating synthetic tissues. - to the original literature
-
2019
Hamashima et al. (2019) extended a sequencing method based on the work of Kimoto et al. (2013) for precise localisation of non-natural base Ds: (7-(2-thienyl)-imidazo-[4,5-b]pyridine) in aptamer-candidates from ExSELEX-libraries (genetic alphabet expansion for systematic evolution of ligands by exponential enrichment). Two different replacement PCRs were used for this in order to exchange non-natural bases with natural bases (N), with the goal of studying the generated products by means of deep sequencing. To be able to draw conclusions on the actual position of Ds, an encyclopedia was created that contained the frequency and composition of the conversion in a specific sequence context (NNNDsNNN). By comparing the current data with the encyclopedia data the position of the Ds could thus be deduced. - to the original literature
Koch et al. (2019) merged DNA molecules with functional materials to generate stable data storage without loss of information (DNA-of-things storage architecture). For this, the DNA molecules were in a capsule o silica nanoparticles embedded in order to prevent a decomposition of the DNA in the production process of the storage. In a proof of principle experiment, DNA encapsulated in silica (SPED) is embedded in polycaprolactone (PCL), a biologically degradable thermoplastic polyester, and printed in the shape of a rabbit by means of 3D-printing. A part of the rabbit was then randomly removed and the DNA located within it was extracted, amplified and decoded. The file stored in the part of the rabbit was able to be reproduced exactly and served as a template to print the F1-generation. Also, no data loss was observed in the following generations up to F5. Following experiments in which the SPED video data was contained and embedded in the most varied material verified the stability of the SPED. A system has thus been created that could be used e.g. for the storage of sensitive data across human generations. - to the original literature
Lee et al. (2019) - The authors extended the knowledge about flexizymes, which can be used to bind novel chemical monomers to tRNAs. The tRNAs bring the monomers to ribosomes, so that these can be incorporated into peptides as non natural amino acids. Lee et al. for the first time produced defined design rules for the novel monomers, so that these can be bound to tRNAs without an effortful trial-and-error process. A plurality of monomers can thus be assembled into peptides hybrid products through ribosomes. As an application of this reprogramming of the genetic code, the synthesis of novel (biological) materials, e.g. high-performance materials, or novel medicines is envisioned. - to the original literature
Zhang et al. (2019) developed a method to secure the data in DNA-data storage, which decouples from the "readout" of the DNA by means of sequencing. The origami cryptography used in the work is based on the self assembly of biomolecules. For the sender to encrypt the DNA-data storage a long scaffold DNA strand is loaded with message strands that contain a biomolecule such as biotin and that differ, depending on the information to be coded, in number and position on the scaffold. Decoding the message by the recipient takes place by means of hundreds of message-specific scaffoldstrands that fold the scaffold into a specific DNA-based nanostructure. This nanostructure is similar to a QR code of square form, but with a pattern similar to Braille writing, comprising numerous points. The orientation of the square that is important for reading the data is assured through a marker DNA strand. This is provided during the encryption by the sender to the messagestrands and is located at a determined position on the scaffold. The data read-out then takes place microscopically with the addition of streptavidin, whereby a Braille point corresponds to a binary number and, in text messages, represents either a letter or its position. For the first time, QR code-like DNA storage has been created that is based on a biomolecule and is cryptographically flexible. - to the original literature
Zhang et al. (2019) - In the field of Synthetic Biology in recent years a considerable quantity of genetic data has been created that, in turn, could be used for the construction of genetic circuits. These data are accessible to the user through various platforms. Sequence-based data, comprising known natural sequences, can be queried via databases with the Basic Local Alignment Search Tool (BLAST). More complex synthetic circuits and designs are in repositories like SynBioHub and are shared publically. For this, SynBioHub uses large datasets of various platforms such as International Genetically Engineered Machine (iGEM). For standardized data exchange the data are coded in the Synthetic Biology Open Language (SBOL). Because the data sets of the various platforms that SynBioHub feeds differ qualitatively and, in part, are deposited translated unstructured in SBOL, search questions and genetic designs are often imprecise. For this reason Zhang et al. implemented organisational technology to sort information from the world wide web in SynBioHub. The goal of the work was to make the search results for search questions on usable circuits and their individual components more precise. Methods were combined with one another for this, establishing the priority of the popularity of the circuit elements. (PageRank) and simplifying the weighting of arising duplicates. (clustering with data infrastructure). The program developed in this work, SBOLExplorer, is implemented as backend service in SynBioHub. The user can therefore compose the circuit graphically as usual via the application SBOL-Designer in SynBioHub and automatically obtains the sorted information for a search question. - to the original literature
-
2018
Bourgeois et al. (2018) developed a CRISPR-Cas9-integration system that uses a series of synthetic DNA landing pads to integrate a number of copies of a DNA into the genome of S. cerevisiae. The pads comprise a specific gRNA-sequences with flanking recombinant regions and function as a zone for multi-copies gene transfer. Varying pads can thereby be present once, twice, three or four times in the yeast genome. It is thereby possible to integrate up to four copies of a gene precisely into the genome of the yeast in only one reaction. In a proof of principle experiment an enzyme (norcoclaurine synthase) with low catalytic activity was selected and was used for the synthesis of benzylisoquinoline alkaloids. By integrating the four copies of the enzyme gene into the landing pads the production of the intermediate molecule ((S)-norcoclaurine) for the synthesis of alkaloids could be doubled. - to the original literature
Synthetic Biology with application perspectives
-
2023
A Dueling-Competent Signal-Sensing Module Guides Precise Delivery of Cargo Proteins into Target Cells by Engineered Pseudomonas aeruginosa (Wu et al. 2023) The authors developed a highly selective platform for protein delivery, called DUEC (dueling competent), by utilising the tit-for-tat/dueling response of the H1-T6SS (type VI secretion system) in Pseudomonas aeruginosa. P. aeruginosa can sense and remember the exact location of physical contact with a neighbouring bacterium and mount a retaliatory response, known as sister cell dueling or tit-for-tat, against heterologous attackers. This unique response in P. aeruginosa is mediated by T6SS, which secretes toxic effectors that kill competitors on direct contact. The authors used DUEC cells, which are P. aeruginosa cells lacking the toxic effectors but are capable of producing H1-T6SS and engaging in dueling. The further developed DUEC cells were able to deliver a nuclease as cargo in a discriminatory manner into the cytosol of T6SS+ but not T6SS- Vibrio cholerae cells to selectively kill provocative cells in a mixed community. Cre recombinase was also used as cargo, demonstrating that DUEC cells are not only a prototypical physical contact sensing and delivery platform, but can also be coupled with recombination-based circuits that have the potential for complex tasks in mixed microbial communities.
A DNA turbine powered by a transmembrane potential across a nanopore (Shi et al. 2024)
The authors generated bottom-up designed nanoscale DNA origami turbines that operate autonomously in physical conditions, converting energy from naturally abundant electrochemical potentials into mechanical work. The turbine contains a central axle with three blades arranged in a chiral configuration, either left or right-handed, and has a height of 24 or 27 nm. It can drive a long DNA bundle as a hydrodynamic load by sustained rotary motion of up to 10 revolutions s−1. The rotational direction of the DNA turbines can be controlled by the ionic strength of the buffer that might be due to a change in the electrophoretic anisotropy ratio with salt concentration.Periplasmic biomineralization for semi-artificial photosynthesis (Lin et al. 2023)
Semiconductor-based biointerfaces are typically established either on the surface of the plasma membrane or within the cytoplasm. Here, the authors have discovered that semiconductor nanocluster precipitates within the periplasmic space of Gram-negative bacteria can efficiently solar-drive chemical production in Escherichia coli. They created nanostructured “exoskeletons” and established semiconductor-based interfaces in the periplasma of E. coli. Specifically, the formation of semiconductor nanoclusters of CdS, one of the most studied optically active materials, was mediated by a H2S-producing nongenetic pathway. Furthermore, they showed that these in situ produced semiconductor nanoclusters could elevate adenosine triphosphate (ATP) levels and enhance malate production under light condition in E. coli. The authors showed that this process of semi-artificial photosynthesis can be applied to the construction of a continuous bioreactor based on living materials for multi-element conversion. The authors suggest that by harnessing the power of biomineralization, and extend it to other bacterial cells, the periplasmic biosynthesis has immense potential for constructing semiconductor-based biohybrids that can be applied in environmental remediation, living bioreactor fabrication, and semi-artificial photosynthesis for bioproduction and various sustainable applications.Sub-1.4 cm3 capsule for detecting labile inflammatory biomarkers in situ (Inda-Webb et al. 2023)
Transient molecules in the gastrointestinal tract are key signals and mediators of inflammation. Owing to their highly reactive nature and extremely short lifetime in the body these molecules are difficult to detect. The authors developed a miniaturized wireless bio-electronic pill, a device that integrates genetically engineered probiotic biosensors (living bacteria) that respond to inflammation-associated molecules like nitric oxide, hydrogen peroxide, tetrathionate, and thiosulfate by producing luminescence, combined with a custom-designed photodetector and readout chip to remotely track these molecules in the gastrointestinal tract. This is realised by transcription of a recombinase in response to the signal biomarker that in turn leads to expression of a luminescence gene in a quantitative manner. Low-power electronic readout circuits integrated into the device convert the light emitted by the encapsulated bacteria to a wireless signal that can be transmitted to a smartphone. All the components were integrated into a sub-1.4 cm3 capsule, compatible to ingestion. The authors demonstrated in vivo biosensor monitoring in the gastrointestinal tract of mice and pigs. With this device, diseases such as inflammatory bowel disease could be diagnosed earlier as currently possible, and disease progression could be more accurately tracked.Cell-free biosynthesis combined with deep learning accelerates de novo-development of antimicrobial peptides (Pandi et al. 2023) bioactive peptides are key molecules in health and medicine with deep learning holding a big promise for their discovery and design. Here, the authors established a high throughput and low-cost approach to identify and validate bioactive peptide candidates within less than 24 h. They used deep learning to design thousands of antimicrobial peptides (AMPs) de novo. Using computational methods, they prioritized 500 candidates that they produced and screened with their newly established cell-free protein synthesis (CFPS) pipeline that rapidly and inexpensively produces AMPs directly from DNA templates. The method takes place in a 384-well setting and the new AMPs are directly tested on 20 μl bacterial cell cultures, followed by OD600 measurement. They identified 30 functional AMPs, which they characterized further through molecular dynamics simulations, antimicrobial activity and toxicity. Notably, six de novo-AMPs feature broad-spectrum activity against multidrug-resistant pathogens and do not develop bacterial resistance.
-
2022
A synthetic transcription platform for programmable gene expression in mammalian cells (Chen et al. 2022) the authors built a synthetic transcription system consisting of CRISPR-based transcriptional activators and guideRNAs (gRNAs). The parts are interchangeable and have been tested both in transiently transfected cells and as stable chromosomally integrated constructs in mammalian cells. Transcription of a gene is controlled by the presence of a gRNA and 2 to 16 complementary binding sites inserted as operators upstream of the gene. A deactivated CRISPR protein fused to an activation domain such as VP16 initiates the transcription. By using different gRNAs, CRISPR transcriptional activators and copy numbers of the gRNA binding sequence as well as through inserting genetic elements to enhance gene expression such as synthetic introns the authors developed promoters with a >1000-fold range of gene expression. The system has been used for the production of a human antibody in CHO cells, in which the genes for the heavy and light chain were each placed under the control of gRNA binding sequences. This approach yielded a high antibody expression and could be applied to produce industrially relevant antibody titres in a controllable and scalable fashion.
Independent control of amplitude and period in a synthetic oscillator with modified repressilator (Zhang et al. 2022) the authors created an oscillator circuit that can be controlled by two inducers in Escherichia coli, one regulating the period of expression, the other the amplitude. They also developed a model for the oscillator that forecasts its regulation. To accomplish the independent control of amplitude and period the circuit was constructed from two plasmids. One contains the repressilator elements and the other the amplitude regulation system. Cells containing the system were placed into a microfluidic system allowing to test eight different concentrations of the inducers at once. Different amplitudes and periods were shown as a function of the relevant inducer concentration. This repressilator could be used, for example, for pulsatile drug delivery.