
Gene Drive Systems - Tools for Accelerated Dissemination of Genetic Modifications
Genetic Basics of CRISPR/Cas9-Based Gene Drive Systems
Since the late 1920s, scientists have been observing that certain characteristics are inherited more frequently in some species than would be expected based on Mendel's laws of inheritance. For example, after mating certain mosquitoes, the progeny can sometimes almost exclusively be male (see Fig.). As the example shows, the distribution of a trait can also be detrimental to the species. The biological mechanisms underlying these types of ‘selfish’ genetic elements or gene drives are manifold.
One of the mechanisms is based on so-called homing endonucleases, which become active in the zygote or later in the germ line. These enzymes are capable of identifying a specific, sometimes unique, sequence in the genome of an organism and introduce a targeted double-strand break in the DNA within it. This strand break can subsequently be repaired naturally in two different ways.
One options is for the two strand ends to be joined together directly. However, this non-homologous end joining often results in the loss of a few base pairs or the incorporation of additional base pairs. The repair can therefore cause a mutation at the site of the original double-strand break.
In contrast, the second mechanism, homology-directed repair, enables less error-prone repair. This type of repair makes use of the fact that the entire genetic information in a cell is usually present in two copies. The double-strand break can thus be repaired by using the copy as a template. However, if an additional DNA sequence is integrated in this template exactly at the broken point, it will be overwritten during the repair to the other copy.
Homing endonucleases can take advantage of this second cellular repair mechanism to propagate the gene coding for them. Provided that the endonuclease gene is flanked by sequences that also occur in the vicinity of the double-strand break, homology-directed repair results in the incorporation of a copy of the endonuclease gene into chromosomes that did not originally contain the gene. If, for example, the information for an endonuclease is originally present on the maternal chromosome, but not on the paternal one, this type of repair results in a copy of the endonuclease gene into the corresponding region of the paternal chromosome following the double-strand break in the paternal chromosome. Since now both chromosomes contain the gene, it is passed on to all offspring. This leads eventually, at least theoretically, to the complete spread of the gene in the population within a few generations.
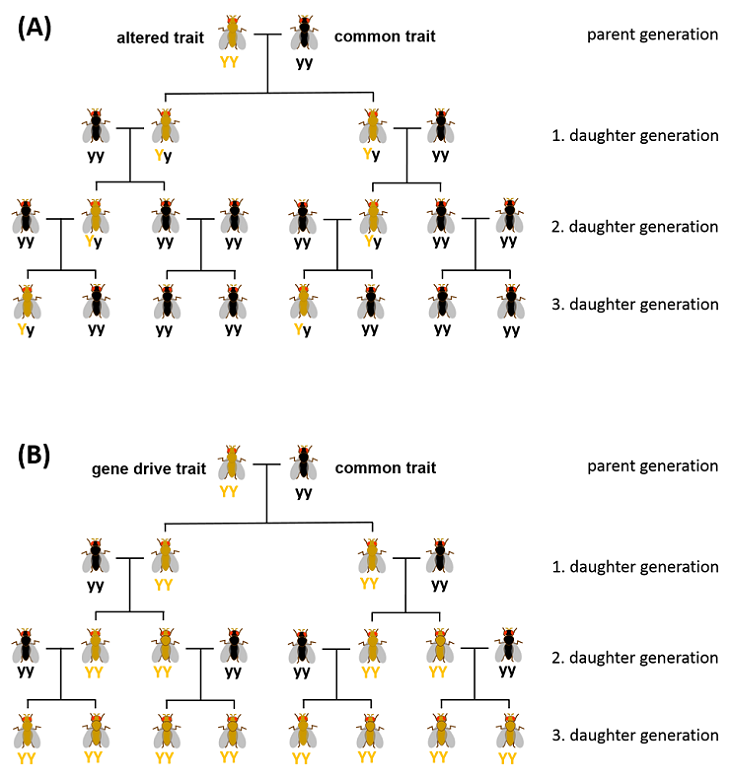
Fig.: Inheritance according to Mendel (A) or of a gene drive (B). The respective genotype, Y (yellow) or y (black), is indicated beneath the flies. (A) The altered trait (yellow) is the dominant phenotype. (B) The altered trait (yellow) is coupled to a gene drive.
Scientists already recognized in the 1960s that the effects of such inheritance via gene drive could be used, for example, to control disease-transmitting mosquitoes. However, the targeted use of gene drives only became technically feasible when the CRISPR/Cas9 tool was developed in 2012, because it made it possible, for example, to generate a double-strand break at a single specific site in the genome. The tool takes advantage of two components of a bacterial virus defence system. The first is an endonuclease (Cas9) and the second a short RNA molecule (guide RNA or sgRNA), which guides the endonuclease to a specific target sequence. The CRISPR/Cas9-based gene drive thus represents a biotechnological imitation of the naturally occurring homing endonucleases described above. Based on today’s state of knowledge, it is the easiest to use gene drive. However, additional systems with other components are also conceivable considering the natural diversity of gene drive mechanisms.
As natural gene drives, also biotechnologically generated gene drives may be used to introduce, modify, or remove a trait of a population. In order to do so, a so-called cargo gene is also passively distributed in addition to the two components of the CRISPR/Cas9 tool. This gene is ultimately responsible for altering a trait of the organism.
Unlike genome editing techniques, which also take advantage of the CRISPR/Cas9 tool, a modification introduced by gene drive is not subject to the Mendelian laws of inheritance. Thus, gene drive and genome editing are fundamentally different, even though they can be based on the same molecular tools. The two methods should therefore not be considered equal.
Applicability of Gene Drive Systems
Generally speaking, there are two distinguishable forms of application of gene drives. On the one hand, a gene drive can be used to deliberately decimate a population. This so-called suppression drive can be achieved by intervening in the sex ratio of the offspring or their fertility. For example, a gene drive could cause infertility in a majority of male offspring or lead to preferential inheritance of one of the two sex chromosomes. The second approach, which can be pursued via gene drives, is the targeted modification of traits of an organism through an alteration drive, without diminishing the organism’s ability to reproduce. In this case, the possibility of preventing the transmission of a particular pathogen is of particular interest. For example, it would be conceivable that a gene is newly introduced into an organism or modified so that a virus can no longer use this organism as a host.
Regardless of the application, a functioning gene drive is subject to certain prerequisites. For example, the target organism has to reproduce sexually. For practical purposes, the generation time of the target organism should also be as short as possible, since it takes several generations for a genetic modification to prevail within a population. In accordance with these conditions, successful application of a gene drive is limited to certain animals and plants. Currently, its main area of envisaged application is in the modification of insect and rodent populations.
Beyond that, other factors may also determine the applicability of a gene drive system. For example, initial laboratory studies have shown that resistance to the CRISPR/Cas9-based gene drive can occur relatively quickly in the target organism. The cause of the resistance is the use of the ‘wrong’ mechanism for the repair of the inserted double-strand break. Specifically, if it leads to non-homologous end-joining instead of homology-directed repair, a mutation is often generated within the recognition sequence of the endonuclease. The sequence is then no longer recognizable and cutable for the endonuclease. The genetic information for the gene drive can therefore not be copied into chromosomes with such a mutation. As a result, the disproportionate inheritance of the gene drive comes to a standstill. Resistance formation would therefore prevent, or at least complicate, the spread of the intended modification within a population. Thus, the gene drive might not achieve the desired effect. The occurrence of resistance mutations varies according to the specific properties of a gene drive system. Frequencies of up to 50% have already been described. This corresponds to the observations of natural gene drive systems, for example in insects where resistance to gene drives has been discovered very frequently.
Two potential areas of application dominate specifically the research and discussion on gene drive systems. In the future, these systems are envisioned to curb insect-transmitted diseases such as malaria, dengue or yellow fever. Both of the approaches described above are pursued to achieve this goal. The goal is to reduce the population sizes of the few mosquito species that transmit the corresponding pathogens. Additionally, the propagation of the pathogens in these species is also supposed to be suppressed. The second area of application is the protection of endangered native ecosystems by controlling invasive animal and plant species. To accomplish this task, suppression drives are used to cause the collapse of the populations of invasive species. It is believed that gene drive systems provide a more effective and targeted solution for conserving natural biodiversity than currently used pesticides, traps and poison baits. However, it should be noted that the question of how to limit a gene drive to a territorial region is currently not resolved.
Assessment of Gene Drive Systems
To function as a gene drive, the genetic information of at least one foreign gene, for example an endonuclease gene, must be introduced into the genome of the parental organism. The resulting organism as well as all other organisms to which the gene drive is transmitted by it, are hence genetically modified. The design and handling of gene drive systems are therefore subject to national and international genetic engineering legislation. So, although it is a comparatively new method, its development processes and products are - contrary to what is often portrayed - already regulated and monitored.
With the amendment of the German Genetic Engineering Safety Ordinance (GenTSV) in 2019, the legislator has stipulated that genetic engineering work aimed at producing genetic elements that drive their own propagation in populations of sexually reproducing organisms must initially be assigned to safety level 3. As a result, work with gene drive systems must undergo a compulsory approval procedure with the respective state authority responsible for genetic engineering. As part of this approval procedure, the state authority is obliged to obtain a recommendation from the Central Commission for Biological Safety (ZKBS) regarding the safety measures that must be observed during genetic engineering work. Since the safety assessment of a gene drive system must take into account criteria that concern not only the genomic modification of the individual, but also, for example, possible environmental effects in the event of an unintended release, an assessment can only be carried out on a case-by-case basis.
Describing the specific project, the ZKBS issues an opinion with a substantiated assignment to a safety level, which may deviate from the preliminary classification in safety level 3. If necessary, it also specifies special technical, organizational or personal safety measures. The case-by-case assessment is based on the intended and possibly unintended consequences of a release of the gene-drive-modified organism, in particular for the environment in its causal network. Notably, the existence of suitable living or reproduction conditions in the receiving environment is used as a basis for evaluation. The case-by-case assessment thus enables basic research on the gene drive topic while simultaneously minimizing the risk of unintentional release.
Gene drive systems are still at an early stage of research, and it will certainly still take some time to answer all of the basic questions for each application. Therefore, experiments are currently only carried out in research laboratories with appropriate safety standards that prevent inadvertent release of gene drive organisms. To clear the path for an actual application, however, experiments under less artificial conditions are indispensable. For example, this can be done in large-scale cage testings, which replicate an ecosystem within a cage that is impermeable to animals. Alternatively, geographically limited releases could also occur on islands. Only when sufficient data has been collected from these trials will it be possible to conceive of approved releases in areas that are not geographically isolated.
So far, one application for a gene drive system for use in a closed research laboratory has been submitted to the ZKBS for evaluation. The system described in this project has proven to induce frequent resistance to the endonuclease used. The gene drive system used therefore poses no danger to humans, animals and the environment, and the project has accordingly been classified to safety level 1.
_____________________________________________________________________________________________
* Working with genetically modified organisms will be assigned to one of four safety levels, depending on their hazard potential for humans, animals and the environment. Safety level 1 is assigned to work with no risk potential, while safety level 4 is assigned to work with a high risk potential. Depending on the assigned safety level, variously strict safety measures must be observed during the work.
A great summary about gene drives can be also found here.
Further reading
- Werren JH (2011). Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc Natl Acad Sci U S A. 108 Suppl 2:10863-70.
Review describing the functioning of different selfish genes. - Thurtle-Schmidt DM, Lo TW (2018). Molecular biology at the cutting edge: A review on CRISPR/CAS9 gene editing for undergraduates. Biochem Mol Biol Educ. 46(2):195-205.
Review summarsing the molecual biological background and possible applications of the CRISPR/Cas9 tool. - Alphey LS, Crisanti A, Randazzo FF, Akbari OS (2020). Opinion: Standardizing the definition of gene drive. Proc Natl Acad Sci U S A. 117(49):30864-30867.
This article points out the importance of consistent definitions of important technical terms in gene drive research.
A list with short definitions of frequently used technical terms can be found here. - National Academies of Sciences, Engineering, and Medicine (2016). Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values. Washington, DC: The National Academies Press.
The US National Academies of Sciences, Engineering, and Medicine describe the basics of gene drive research and illustrate examples. Furthermore safety related and ethical questions are discussed. - Raban RR, Marshall JM, Akbari OS (2020). Progress towards engineering gene drives for population control. J Exp Biol. 223(Pt Suppl 1):jeb208181.
This review article introduces different types of gene drives. - Esvelt KM, Smidler AL, Catteruccia F, Church GM (2014). Concerning RNA-guided gene drives for the alteration of wild populations. eLife. 3: e03401.
Review summarising the chances and limitations of gene drive systems as well as possible safety measures. - Gantz VM, Bier E (2015). The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science. 348(6233): 442–444.
The artcicle describes the development of the first gene drive in fruit flies. - Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA (2015). Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A. 112(49): E6736–E6743.
The artcicle describes the development of a gene drive in the Malaria vector Anopheles stephensi. - Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S, Burt A, Windbichler N, Crisanti A, Nolan T (2016). A CRISPR-Cas9 Gene Drive System Targeting Female Reproduction in the Malaria Mosquito vector Anopheles gambiae. Nat Biotechnol. 34(1): 78–83.
The artcicle describes the development of a gene drive in the Malaria vector Anopheles gambia. - de Jong TH (2017). Gene drives do not always increase in frequency: from genetic models to risk assessment. J Consum Prot Food Saf. 12: 299.
Based on mathematical models this article regards different gene drive systems and the likelihood of their establishment in natural populations. - KaramiNejadRanjbar M, Eckermann KN, Ahmed HMM, Sánchez C HM, Dippel S, Marshall JM, Wimmer EA (2018). Consequences of resistance evolution in a Cas9-based sex-conversion suppression gene drive for insect pest management. Proc Natl Acad Sci U S A. 115(24):6189-6194.
This article describes the development of resistance against a gene drive in fruit flies. - Akbari OS, Bellen HJ, Bier E, Bullock SL, Burt A, Church GM, Cook KR, Duchek P, Edwards OR, Esvelt KM, Gantz VM, Golic KG, Gratz SJ, Harrison MM, Hayes KR, James AA, Kaufman TC, Knoblich J, Malik HS, Matthews KA, O'Connor-Giles KM, Parks AL, Perrimon N, Port F, Russell S, Ueda R, Wildonger J (2015). Safeguarding gene drive experiments in the laboratory: Multiple strategies are needed to ensure safe gene drive experiments. Science. 349(6251): 927–929.
This article proposes safety measures on different stages for the handling of organisms that have been modified by gene drives. - Buchman A, Marshall JM, Ostrovski D, Yang T, Akbari OS (2018). Synthetically engineered Medea gene drive system in the worldwide crop pest Drosophila suzukii. Proc Natl Acad Sci U S A. 115(18):4725-4730.
This article describes a gene drive based on the toxin-antitoxin system »Medea«. - Zhang T, Mudgett M, Rambabu R, Abramson B, Dai X, Michael TP, Zhao Y (2021). Selective inheritance of target genes from only one parent of sexually reproduced F1 progeny in Arabidopsis. Nat Commun. 12(1):3854.
This article is the first to describe a gene drive in a plant.
published: July 2018, updated december 2021