Statistics
Approval of genetic engineering facilities
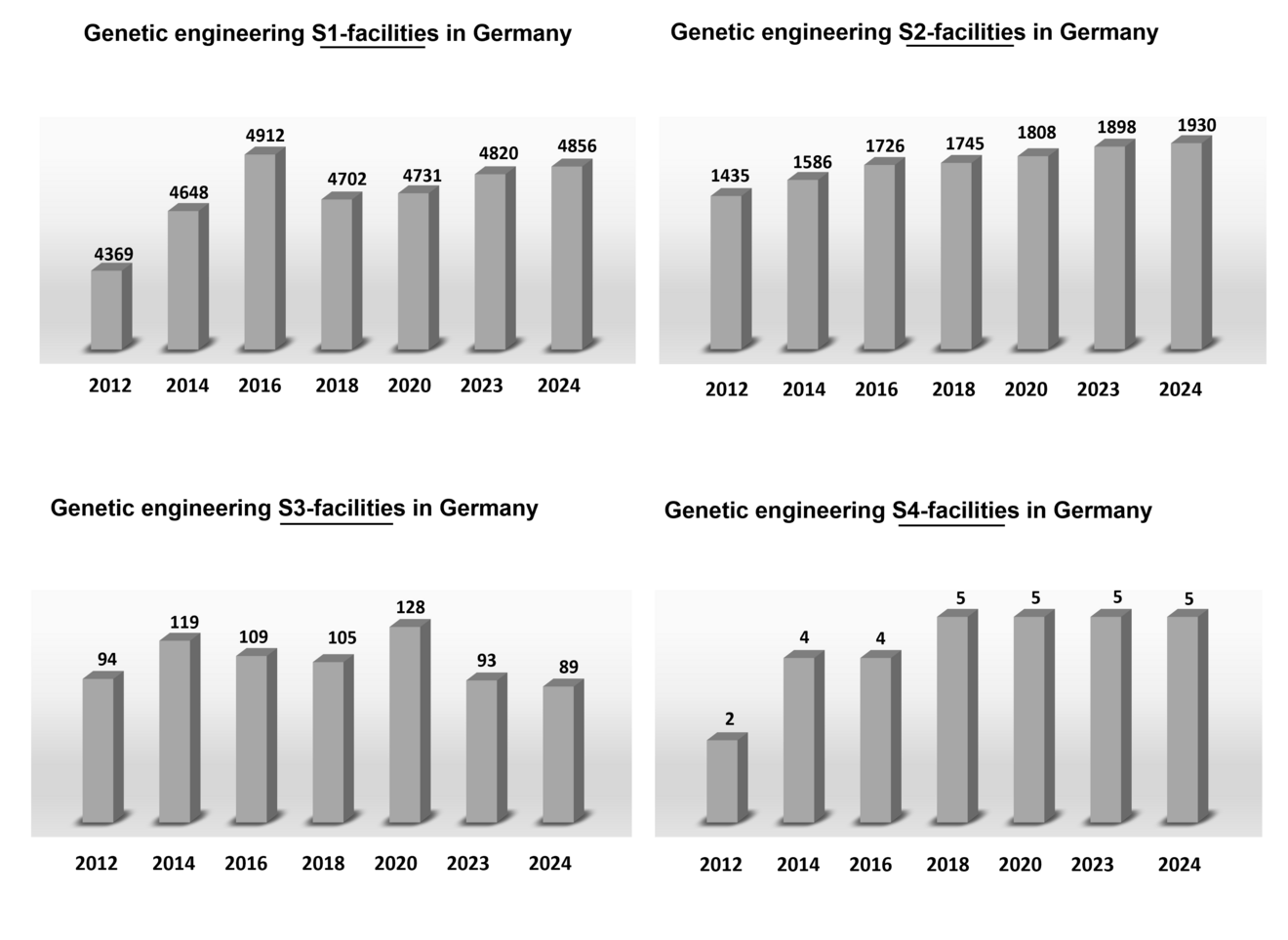
Based on the reports from the federal states, two overview graphics are shown below for the genetic engineering facilities approved in Germany within the respective security/safety level. The actual number of genetic engineering facilities with security/safety level 1 and 2 may differ slightly from the number mentioned.
![[Translate to English:] Graphic summarizing the number of genetic engineering facilities of security level S1, S2, S3 and S4 in Germany as of December 2023.](/fileadmin/_processed_/6/5/csm_Abb._1_Gent._Anlagen_in_D_2024_EN_216ca914ee.png)
Displayed, registered or approved genetic engineering facilities in Germany (as of December 2023).

Overview of the number of genetic engineering facilities of security levels 1 to 4 in the years 2012 to 2023.
Approval of genetic engineering work
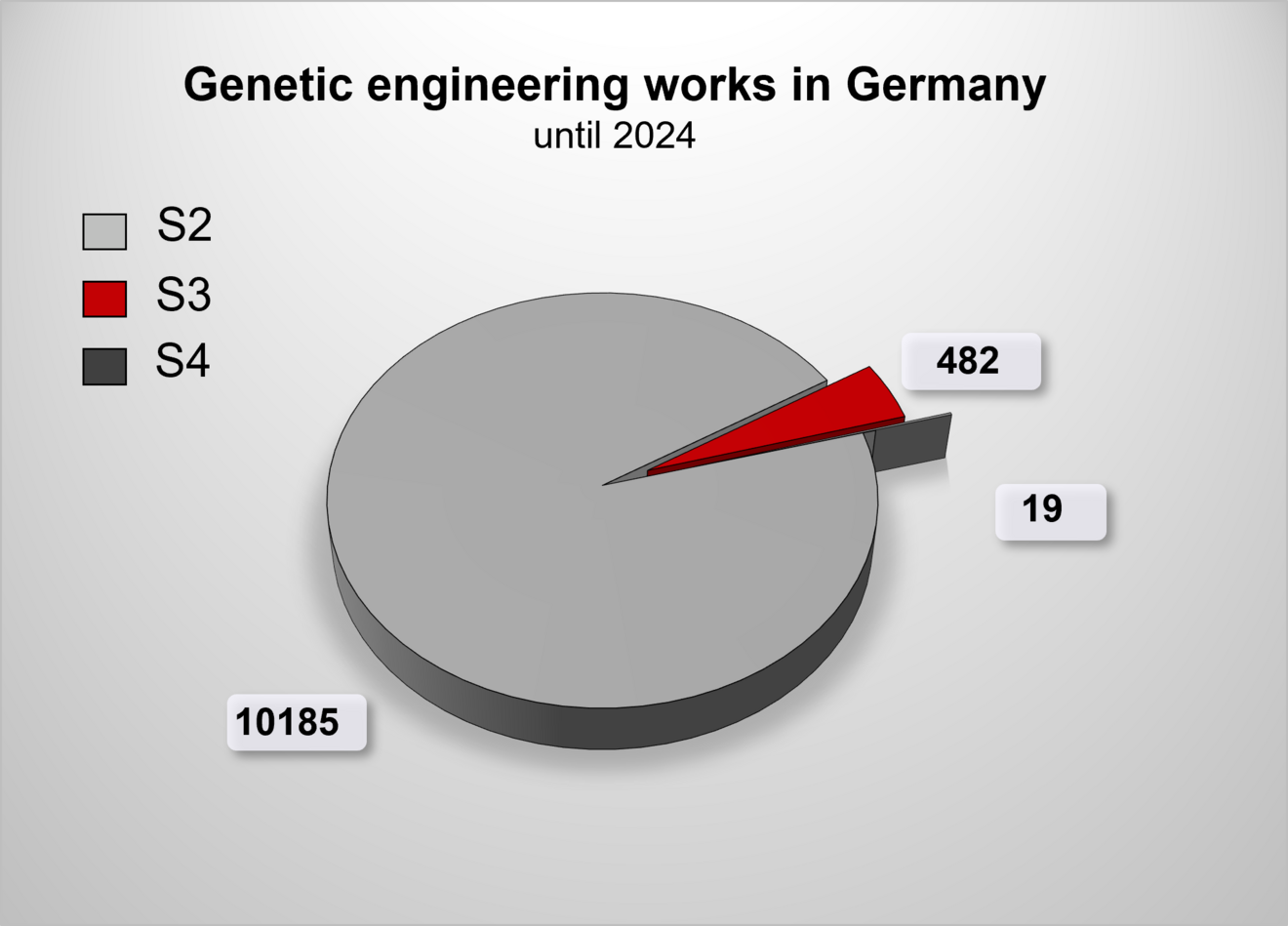
The following graphic summarizes the genetic engineering work that is displayed, registered or approved in Germany, depending on the security/safety level.
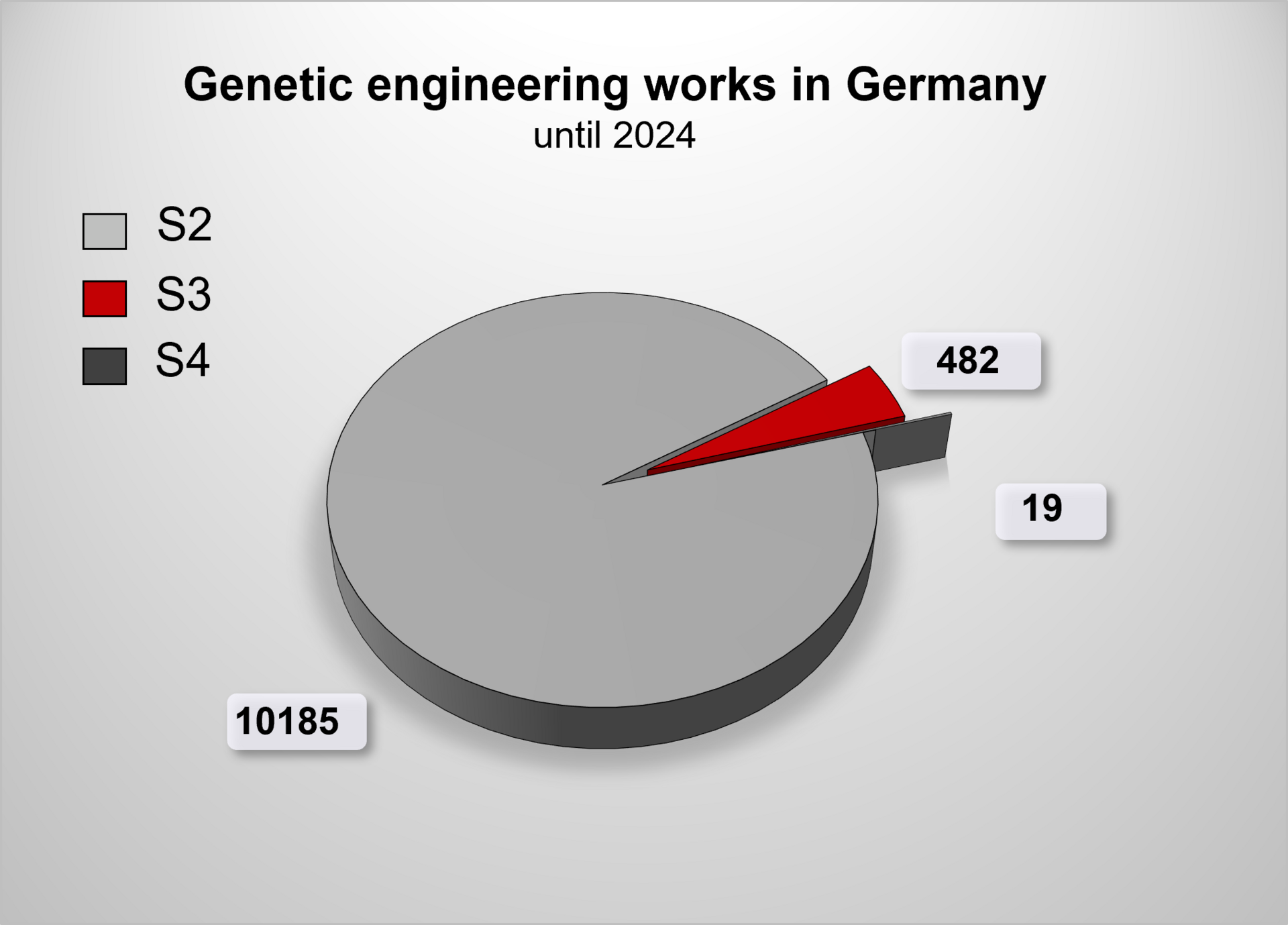
Overview of genetic engineering operations in Germany 2024. It is not possible to provide a precise figure for the genetic engineering operations carried out at safety level 1, because the operators are obliged to keep records of further safety level 1 operations in accordance with § 9 GenTG, but there is no obligation to notify or report them to the competent state authority. Thus, further S1 operations are not recorded in the official databases.
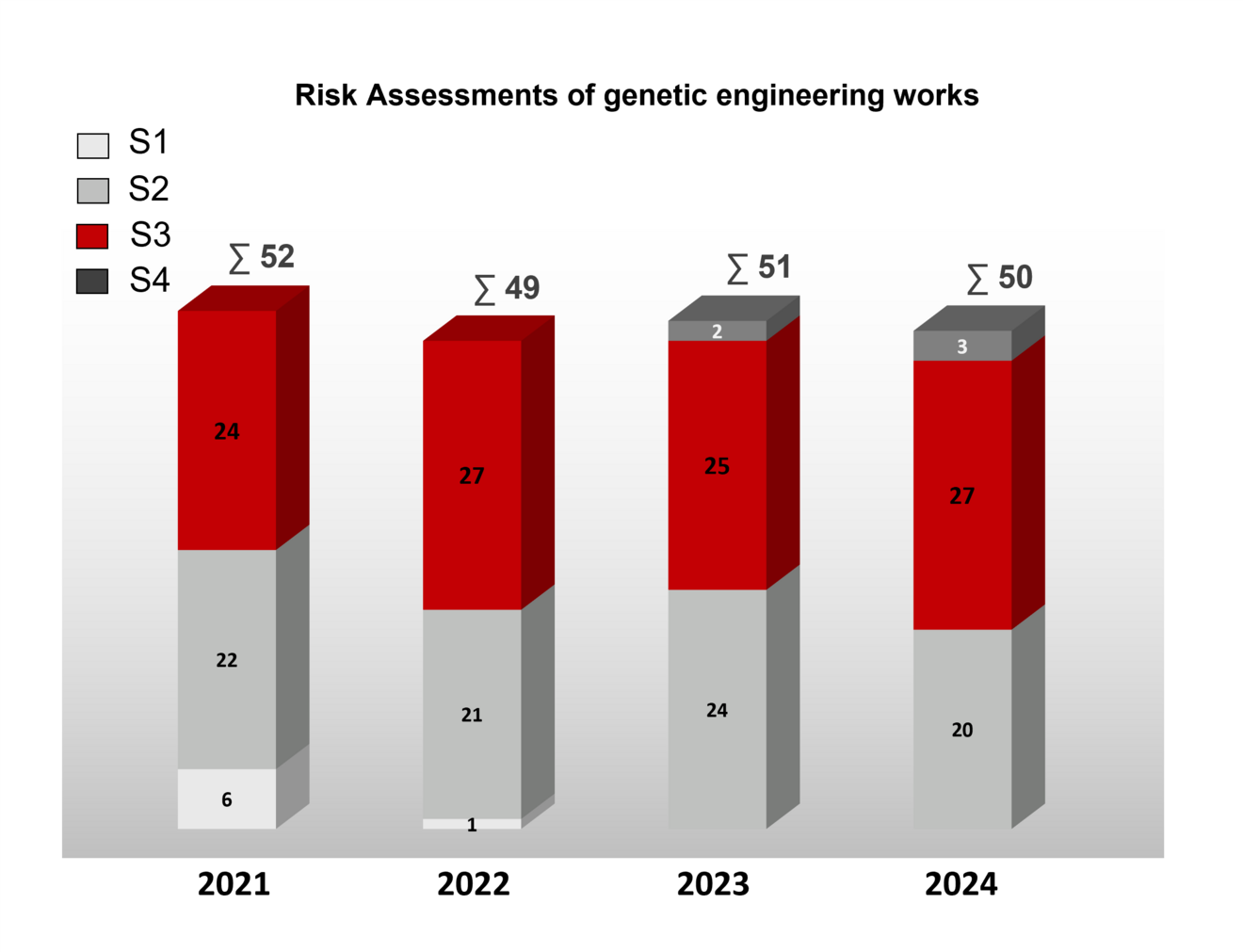
The following is an overview of the safety classifications of genetic engineering work and assessments of safety-related measures of genetic engineering facilities by the ZKBS in recent years.
For most of the genetic engineering work that was assessed, there was only a reference to the GenTSV for the safety measures. For some, however, a detailed assessment was made.
The statements/recommendations of the ZKBS on specific projects are not published for data protection reasons and are only available to the competent approval and monitoring authorities of the federal states.
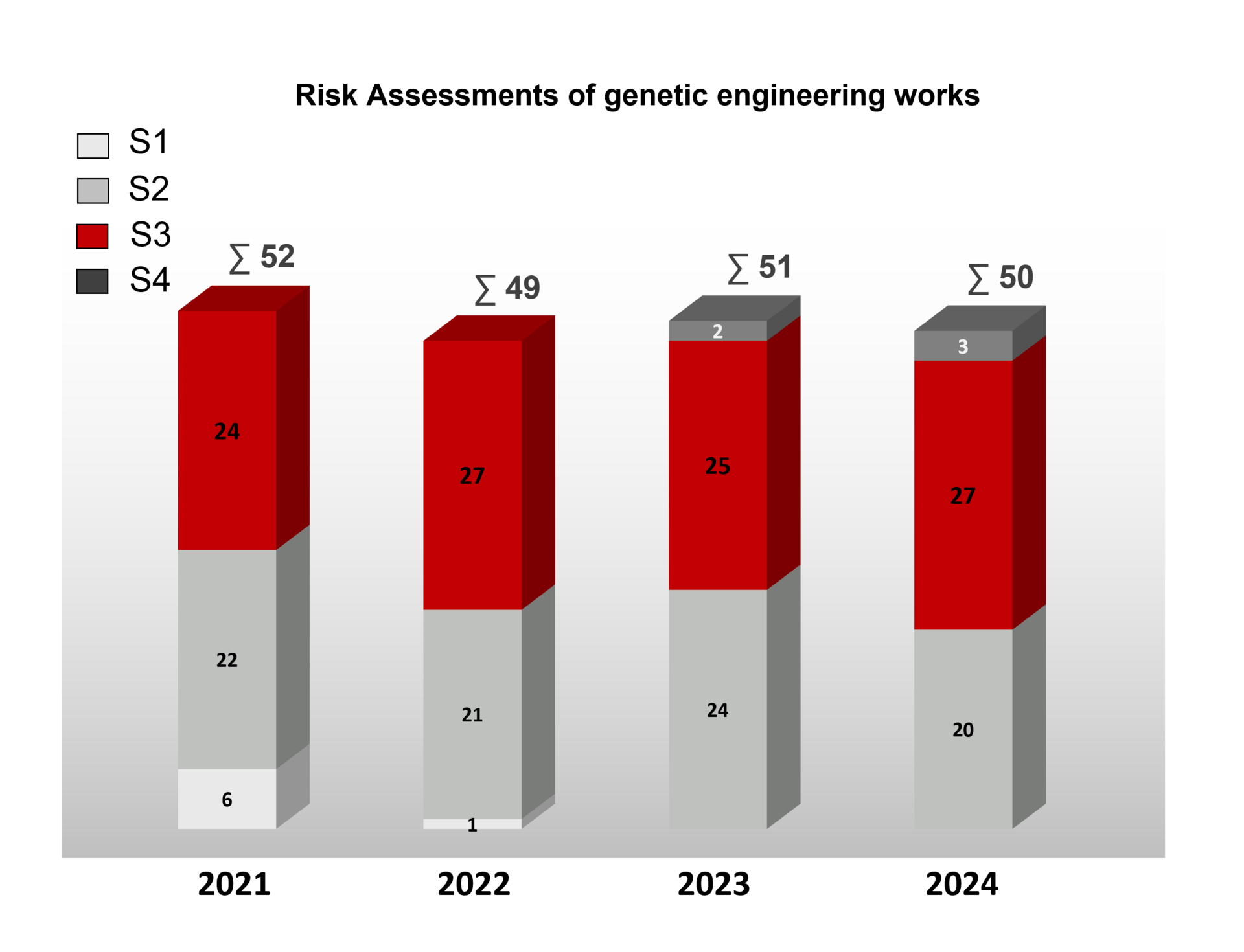
Overview of the number of genetic engineering work assessed by the ZKBS at safety levels (S) 1 to 4 in the years 2020 - 2023. The total number of genetic engineering work assessed in the respective year is indicated after the sum symbol ∑. The majority of genetic engineering work at security level 1 and 2 is approved without the involvement of the ZKBS.
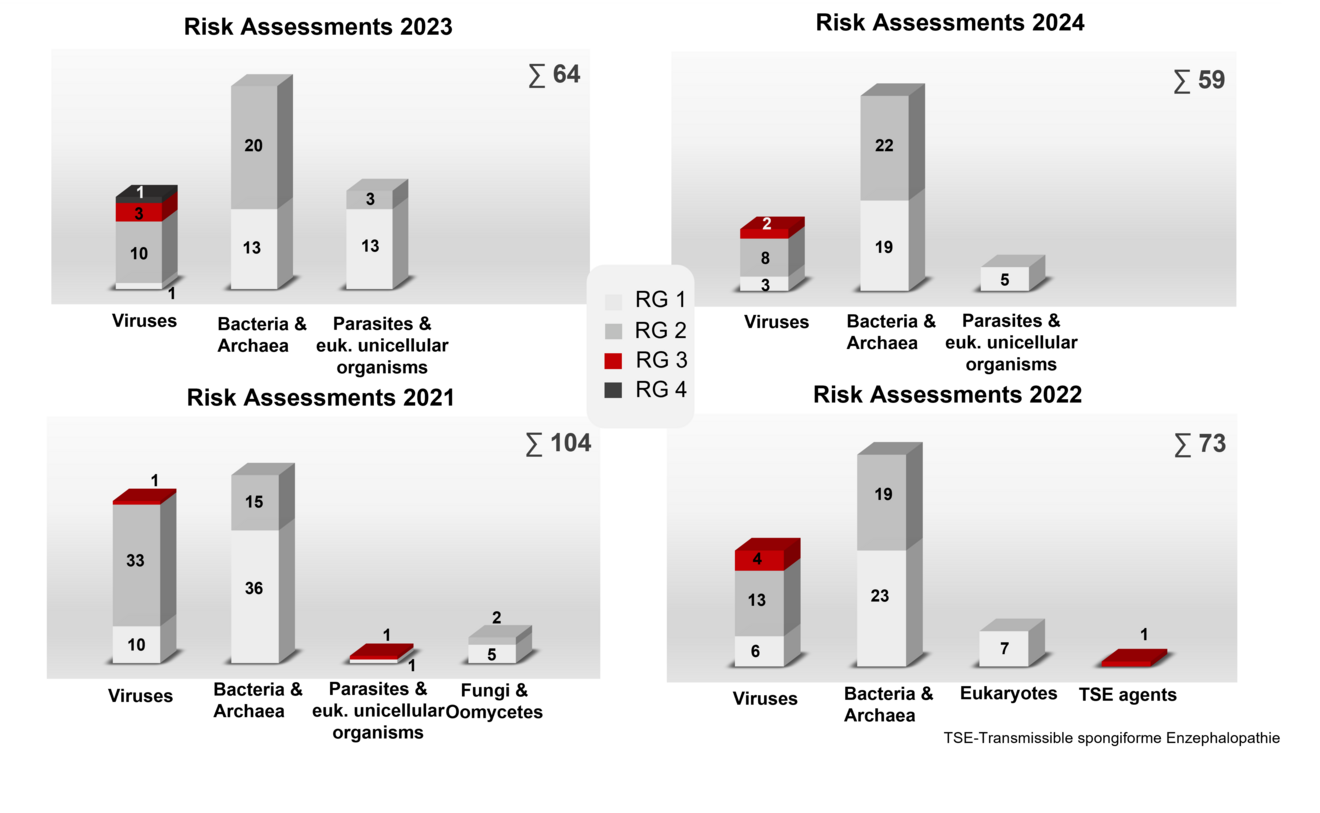
Risk assessment of donor and recipient organisms
The following microorganisms, which are used as donor or recipient organisms in genetic engineering, were assigned to a risk group in the year mentioned in accordance with § 5 and with Appendix I GenTSV.
You can find the risk assessments in the organisms database. For many classifications there is a general statement/recommendation in which the risk assessment can be looked up.

Overview of the type and number of donor and recipient organisms of risk groups (RG) 1 to 4 that have been risk-assessed by the ZKBS in the years 2020 - 2023. The total number of organisms assessed in the respective year is indicated after the sum symbol ∑. euk. - eukaryotic
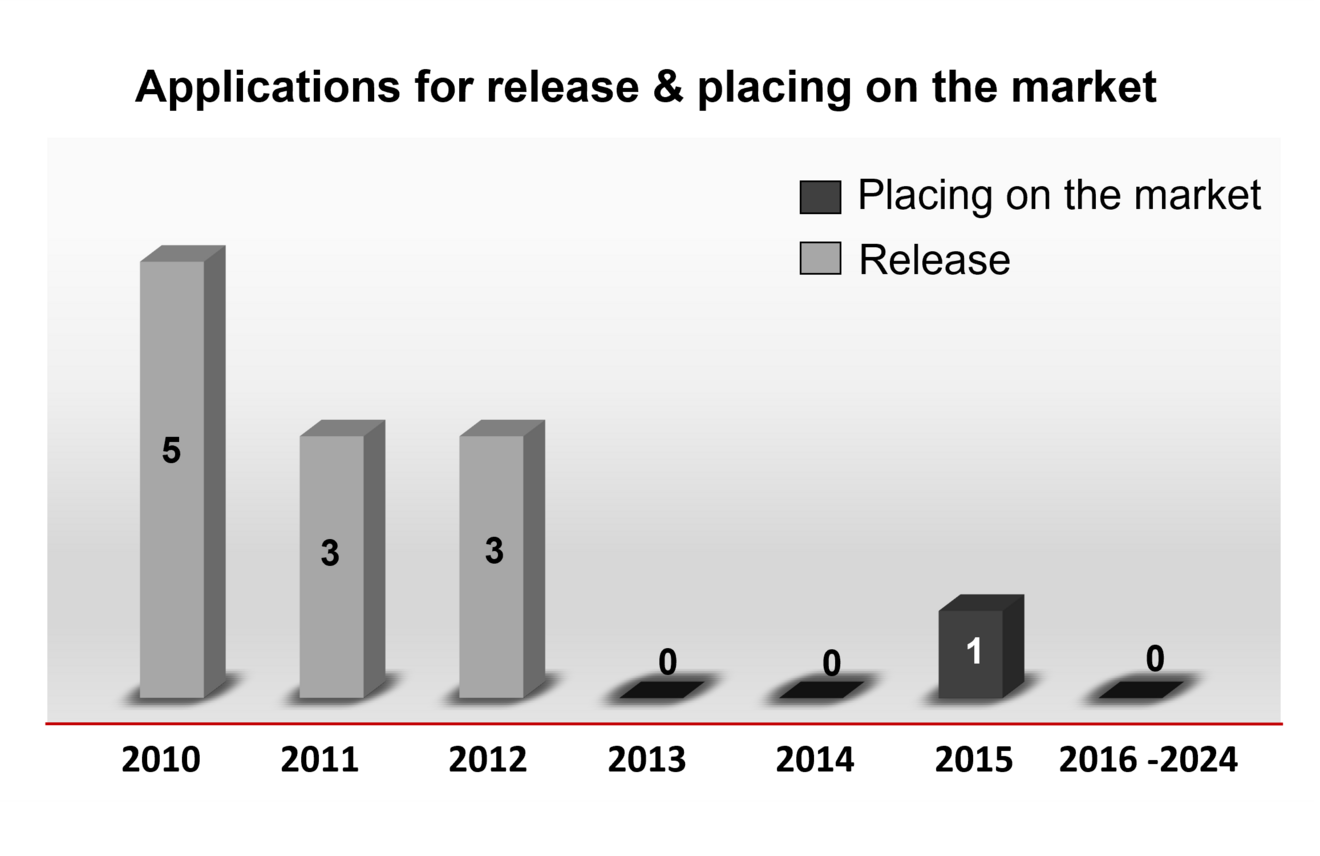
Statements/Recommendations on releases and placing on the market
In the following you will find an overview of the number of statements on releases and placing on the market that were issued by the ZKBS in the respective year.
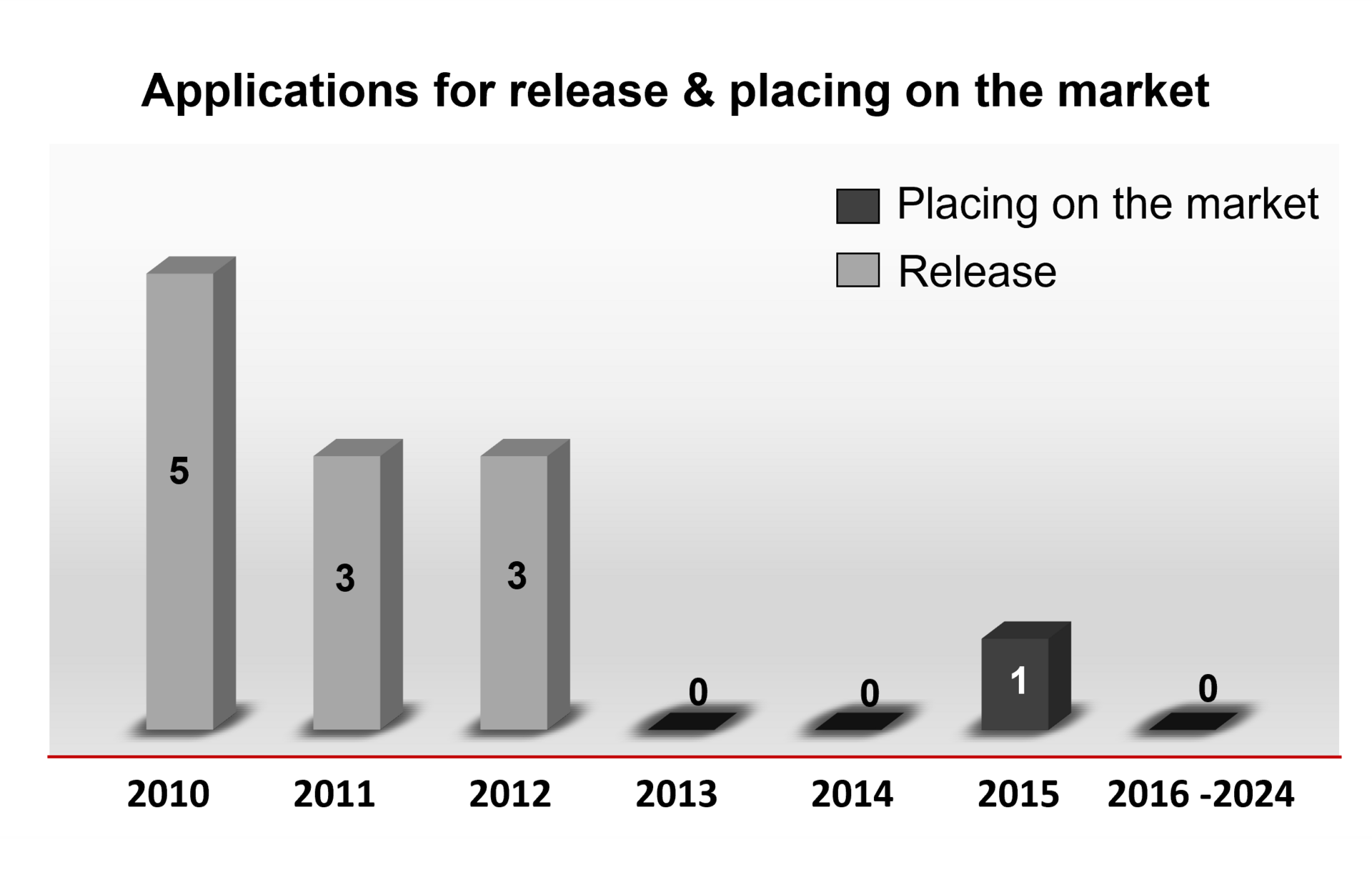
Overview of the number of statements by the ZKBS on applications for release and placing on the market in the years 2010 to 2023.
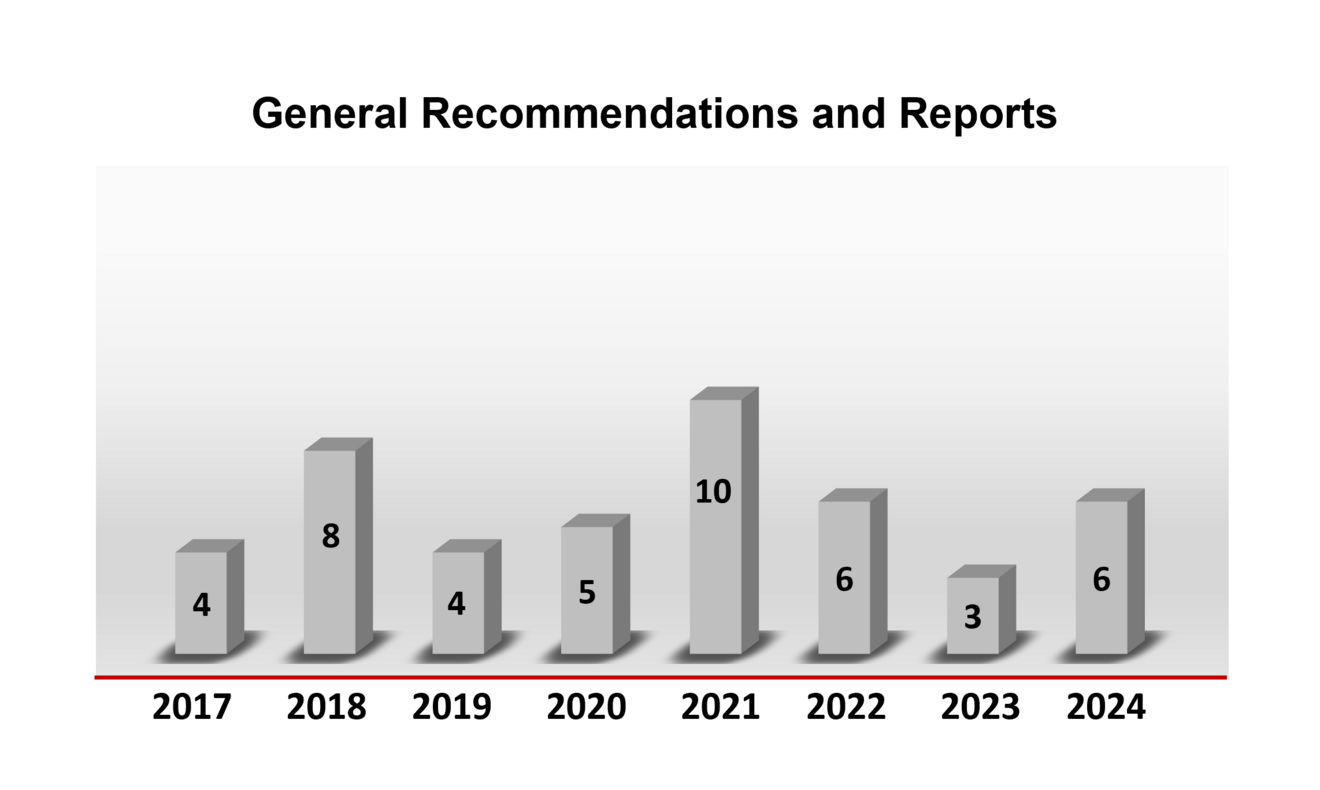
General recommendations
In the following you will find an overview of the number of general statements issued or updated by the ZKBS in the respective year.
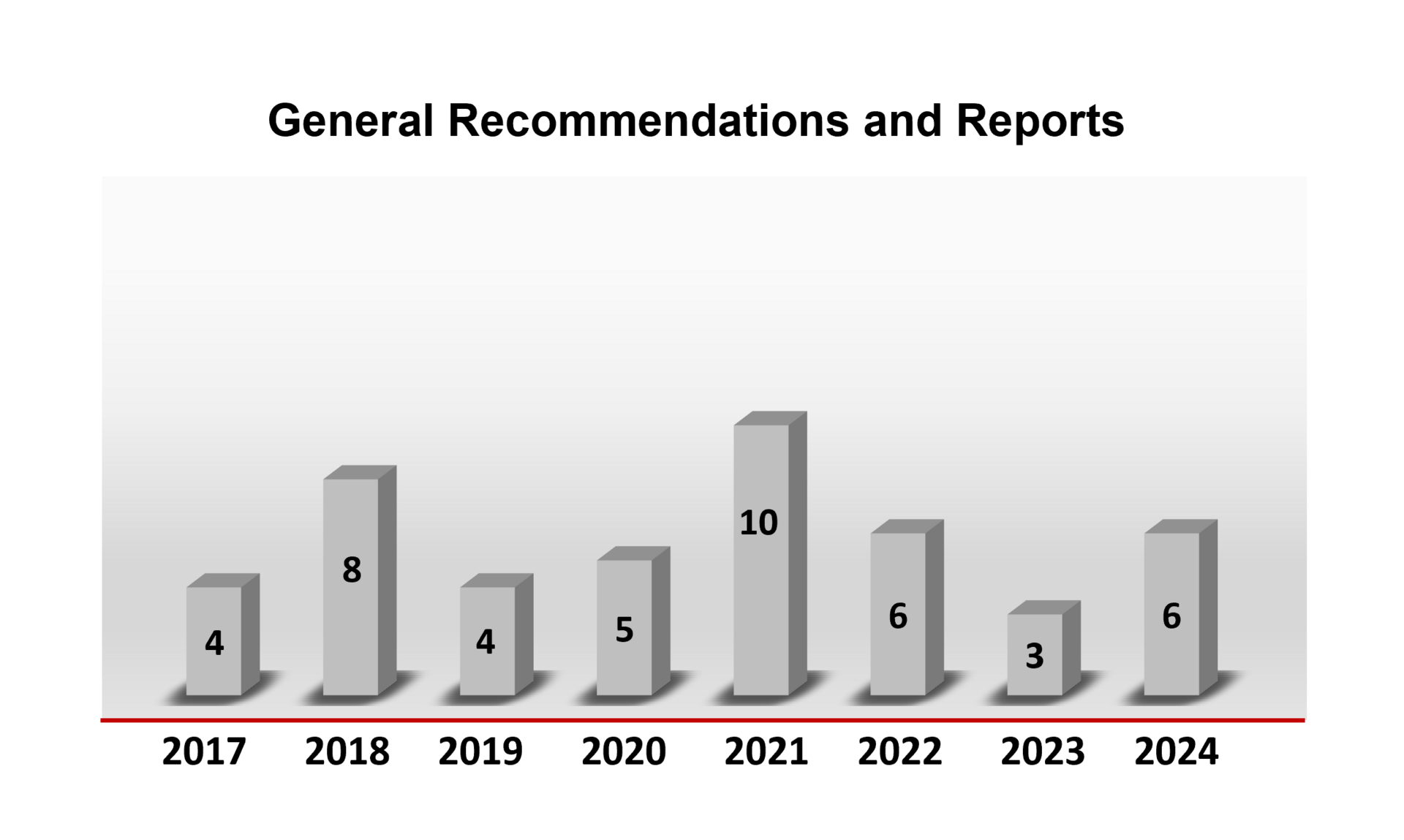
Overview of the number of general statements by the ZKBS in the years 2016 to 2023.
You can find all general statements here.
![[Translate to English:] Graphic summarizing the number of genetic engineering facilities of security level S1, S2, S3 and S4 in Germany as of December 2023.](/fileadmin/_processed_/6/5/csm_Abb._1_Gent._Anlagen_in_D_2024_EN_d533761568.png)