
Influenza viruses
Influenza viruses – causative agents of the ‘real’ flu
The World Health Organization estimates that three to five million people worldwide develop a severe form of influenza every year. An estimated 290,000 to 650,000 of these infections are fatal. In Germany, up to 20,000 people die from the disease in a flu season. But even less dramatic courses of the disease cause enormous economic damage. For example, the Robert Koch Institute estimates that 5 – 20% of the German population get infected during the approximately eight- to ten-week long annual flu epidemic. The loss of working hours and costs for the public healthcare system are correspondingly high.
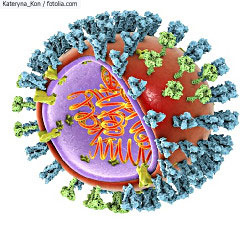
Schematic representation of an influenza virus particle
The common flu (influenza) is caused by influenza A and B viruses. It is often difficult to differentiate from flu-like illnesses caused by other viruses based on symptoms alone. Influenza is characterised by a sudden onset of symptoms such as fever, cough, headache, muscle and joint pain as well as general fatigue. Most people recover from the disease within a week. However, the infection can be severe and require medical treatment – especially in older people and in people with underlying conditions or a weakened immune system.
In addition to influenza A and B viruses, there are also influenza C viruses that can infect humans. However, they seem to cause only very mild symptoms – if any at all. Antibodies against influenza C viruses are widespread throughout the population, which suggests that infection with these viruses often goes unnoticed. Consequently, the viruses have a rather minor impact on public health. Lastly, there are also influenza D viruses that infect cattle, sheep, goats and pigs. Human infections are not known yet. Influenza A viruses can also infect various animals. However, these infections are usually followed by a mild course of the disease. One exception are infections in domestic poultry, where certain influenza A viruses can cause the fatal avian flu.
Influenza viruses are highly contagious and are transmitted via the air by droplets, such as those produced from coughing, or by contact with contaminated surfaces such as door handles and hands. The viruses can also remain infectious on these surfaces for up to eight hours at room temperature and up to 30 days at temperatures around the freezing point. Therefore, the most effective protection against infection is proper hand hygiene and protective vaccination, which has been available for more than 60 years (more information on influenza vaccines and be found here soon). The time between infection and the appearance of the first symptoms of the disease is called the incubation period and lasts one to four days.
Masters of variation
The genetic information (the genome) of influenza A and B viruses is divided into eight parts called genome segments. This genetic information is subject to constant change. On the one hand, reading errors occur when it is amplified during the reproduction cycle of viruses. On the other hand, if a cell is infected with two or more different influenza viruses at the same time, the genome segments can be can be combined anew. Both of these factors lead to the very rapid development of viruses with new characteristics. Of particular importance here is the adaptation to a new host and the circumvention of the human immune system.
To account for the diversity of viruses, influenza A viruses are divided into subtypes. The classification is based on the viral surface proteins haemagglutinin (HA, 18 types) and neuraminidase (NA, 11 types), whose combination determines the subtype of the viruses. For example, viruses of subtype H3N2 consist of type 3 HA and type 2 NA. Analogously, influenza B viruses are classified into the Victoria line and the Yamagata line based on their HA. Despite this rough classification, viruses of the same subtype or line can cause illnesses of varying severity. This is due to the variability of the other viral proteins.
The viruses that currently cause seasonal flu in humans are classified as influenza A viruses of subtypes H1N1 or H3N2 or one of the two lines of influenza B virus. So far, influenza A viruses of other subtypes have occurred almost exclusively in animals. However, some of them can also occasionally infect humans and cause severe, often fatal, diseases. The best known examples of such sporadically infecting viruses are viruses of subtype H5N1. They originate naturally from birds, but can be transmitted to humans through close contact. Infections with these viruses are fatal in half of the cases.
The surface proteins are also recognised by the immune system. Although the antibodies formed against the surface proteins are very efficient in defending against the viruses, they are also very specific. This means that they only recognise those viruses that are present in the current infection. Since the viruses are very likely to exhibit a large variety of changes during the next flu season, the previously formed antibodies are largely ineffective. Therefore, the immune response against the new viruses must be built from scratch. In order to ensure customised protection, vaccination must thus also be performed annually with the virus strains that are predominant at that time. As a result, a new vaccine cocktail must be created every year.
If the surface proteins have changed to such a great extent, for example by a new combination of genome segments, that the antibodies already present in the population do not provide protection, a global spread (a so-called pandemic) of the influenza virus in question may occur. In contrast to seasonal influenza waves, which are triggered by regionally varying strains, in this case a single virus strain and its progeny lead to a global influenza outbreak. So far, four influenza outbreaks have been declared pandemics by the World Health Organization. All four were caused by influenza A viruses. The first one was the ‘Spanish flu’ (subtype H1N1) in 1918 with about 20 to 50 million deaths, followed by the ‘Asian flu’ (subtype H2N2) in 1957 with about two million deaths, the ‘Hong Kong flu’ (subtype H3N2) in 1968 with about one million deaths and the ‘Mexico flu’ (also known as ‘swine flu’) (subtype H1N1) in 2009 with 150,000 to 575,000 deaths. As the last pandemic showed, pandemics do not necessarily have to be accompanied by a high number of fatal infections (cf. number of deaths in a ‘normal’ flu season). However, even a pandemic with a comparatively mild course can overload national health care systems due to the high amount of cases of illness. Moreover, the severity of a future pandemic cannot be predicted. Therefore, it is important to take early precautions for the event of a pandemic (more information on the German pandemic plan can be found here soon). A key part of this is research into currently circulating viruses in order to be able to assess the dangers posed by new viruses and to detect viruses with pandemic potential at an early stage. Particular attention is being paid to influenza A viruses of the H5 and H7 subtypes, which have already been repeatedly transmitted from birds to humans and have frequently caused deadly infections in humans. So far, these viruses are not efficiently transmitted from person to person. Should this change in the future, a devastating pandemic may well be the result.
Genetic engineering operations with influenza viruses
Genetic engineering operations with influenza viruses are carried out to investigate a wide range of questions. For example, genetic engineering can help elucidate the response of the human immune system to an influenza virus infection. It can also help develop new antiviral agents and vaccines and lead to a better understanding of the continuous development of circulating influenza viruses. The latter mostly aims to identify changes in the viral genetic information that enable the virus to multiply more efficiently in humans, to better circumvent the human immune system or to be transmitted from animals to humans. This information can then be used to predict the characteristics of new viruses and thus to make early preparations in case of a new influenza pandemic.
To achieve these goals, researchers can use two different approaches: Reassortment and mutagenesis. Reassortment (Fig. 1) involves targeted generation of novel influenza viruses by introducing individual genome segments of different influenza viruses into a host cell and combining them to form a new set of eight genome segments. These types of experiments can provide information on the general significance of certain variants of the genome segments and the proteins encoded by them for the reproduction of a virus, its transmissibility or health hazards.
Figure 1: Creation of novel influenza viruses through reassortment. If a host cell is naturally infected with more than one influenza virus, the progeny of the viruses each contain a random set of the eight viral genome segments (selection shown as an example) (A). For the targeted generation of novel influenza viruses in the laboratory, the genome segments are first isolated and introduced into host cells in a desired combination (B).
By contrast, mutation experiments (Fig. 2) can be used to precisely trace the cause of an observed change to individual protein building blocks. For this process, random or very precise changes (so-called mutations) are introduced into the genetic information of a virus either naturally or with the aid of molecular biological techniques. These changes are then linked to changes in the properties of the virus (more information on mutation experiments can be found here soon).
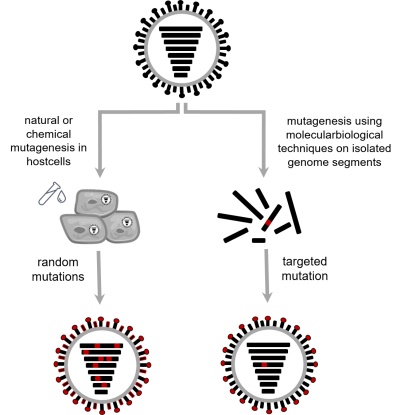
Figure 2: Creation of novel influenza viruses by mutagenesis. When viruses multiply in a host cell, mutations in the viral genetic information are created. By adding certain chemical agents, the number of these natural mutations can be increased. Their quantity, type and position cannot be predicted. However, with the help of molecular biological techniques on isolated genome segments, one or more precisely defined mutations can be inserted into the genetic information.
Risk assessment of genetic engineering operations with influenza viruses
In Germany, the evaluation criteria for genetic engineering operations with microorganisms, viruses and eukaryotic protozoa in closed systems (e. g. laboratories) are listed in the Genetic Engineering Safety Regulations (GenTSV). The objective of the assessment is to establish sufficient safety measures for the realization of genetic engineering operations that consider the risks that a genetically modified organism could pose to the health of humans and animals, to the environment in general and to other legal interests. At the same time, however, the regulatory requirements imposed on the realization of genetic engineering operations must also enable and promote innovations in the fields of biotechnology, plant cultivation and, in particular, medicine in Germany. To this end, it must be assessed whether and to what extent a particular genetic modification could alter the biological properties of an organism and thus its natural hazard potential. This information can then be used to derive the potential damage and its severity and to make predictions about the probability of this damage occurring. The combination of the theoretical amount of damage and its occurrence probability is generally referred to as the risk of a project. Depending on the identified risk, variously strict safety measures must be observed during the genetic engineering operations. They can be higher, lower or just as high as the safety measures required for work with the non-genetically modified starting organism. The exact requirements for these measures are laid down in the GenTSV. Compliance with these regulations is monitored by the competent state authorities.
In addition to position statements on specific projects, the German Central Committee on Biological Safety (ZKBS) also issues position statements of a general nature. These position statements evaluate the organisms that are to be used for genetic engineering operations and define generally applicable further evaluation criteria for frequently carried out genetic engineering operations.
A prominent topic of such general position statements are influenza A viruses or work with these viruses. The reason for this is that a large number of different influenza A viruses occur in nature, all of which can cause diseases of varying severity in humans and animals. The cause of these differences is not always easy to identify. Moreover, due to the rapid development of influenza viruses, in nature or in the laboratory, virus variants occur constantly that can pose a different risk to humans, animals and the environment compared to their closest relatives.
In order to arrive at comprehensible and justified assessments, even in light of this complexity, the German Central Committee on Biological Safety used the extensive knowledge already published in the specialist literature to develop largely generally applicable criteria for the assessment of typical genetic engineering operations with influenza A viruses. If it cannot be assumed that these criteria are generally applicable, the German Central Committee on Biological Safety carries out case-by-case assessments. Furthermore, if the data are unclear and do not permit a sufficiently reliable assessment of the risk of an operation, a precautionary classification in the higher possible biosafety level is always selected. Like all recommendations by the German Central Committee on Biological Safety, these classifications and their criteria are reviewed as soon as new scientific data become available and the position statements are subsequently updated if necessary.
Links to position statements of the ZKBS regarding influenza A viruses
- General recommendations regarding genetic engineering operations with influenza A viruses
- Position statement of the ZKBS on the risk assessment of genetic engineering operations with recombinant influenza A viruses (Ref. No.45310.0113 (not accessible) [PDF, 883KB] , updated December 2019)
- General position statement of the ZKBS on the classification of genetic engineering operations with highly pathogenic avian influenza A viruses (HPAIV) which possess the potential for efficient airborne transmission between mammals (Ref. No. 45310.0108 (not accessible) [PDF, 59KB] , March 2013)
- Risk assesement of influenza A viruses as donor or recipient for genetic engineering operations
- Position statement of the Central Committee on Biological Safety on the risk assessment of influenza viruses (Ref. No. 6790-05-02-29 (not accessible) [PDF, 18.54KB] , updated November 2015)
- Position statement of the ZKBS on the risk assessment of Highly pathogenic avian Influenza virus A strains of the subtype H5 and H7 and derived laboratory strains according to Sec. 5 (1) GenTSV (Ref. No. 6790-05-02-34 (not accessible) [PDF, 58KB] , updated July 2015)
- Recommendation of the ZKBS on the risk assessment of influenza A viruses of subtype H5N8 as donor or recipient organisms according to Article 5 paragraph 1 GenTSV (Ref. No. 45242.0145 (not accessible) [PDF, 298KB] , July 2017)
- Update of the recommendation of the ZKBS on the risk assessment of novel avian influenza A virus H7N9 as donor or recipient organism for genetic engineering operations according to Article 5 paragraph 1 GenTSV (Ref. No. 45242.0103 (not accessible) [PDF, 374KB] , September 2013)
- Recommendation of the ZKBS on the risk assessment of influenza A virus strains SC35 and SC35M (A/Seal/Massachusetts/1/80) as donor or recipient organisms according to Article 5 paragraph 1 GenTSV (Ref. No. 45242.0116 (not accessible) [PDF, 293KB] , July 2015)
- Statement of the ZKBS on the evaluation of the influenza A virus mutant „Delta NS1” (Ref. No. 6790-10-71 (not accessible) [PDF, 313KB] , September 2001)
published May 2019